http://www.chemistrymag.org/cji/2005/077048ne.htm |
Jul. 2, 2005 Vol.7 No.7 P.48 Copyright |
Synthesis and characterization of the octaaminopropyl-silsesquioxane
Gao Jungang*, Zhang Xuejian, Wang Shichen,
Run Mingtao
(College of Chemistry and Environmental Science, Hebei University, Baoding, 071002, China)
Received Apr. 18, 2005
Absract Octaaminopropylsilsesquioxane (OAPS) was synthesized with
alkali-equilibrated mixtures of
Keywords Synthesis, Octaaminopropylsilsesquioxane, Characterization
1. INTRODUCTION
In the field of inorganic-organic hybrid materials, there is growing interest in polyhedral silsesquioxane-based systems. The interest of these silica-like cage structures is driven by both their growing commercial availability and the relative ease of attaching functional groups to the cage, facilitating the incorporation of the organic component into a macromolecular system.
Polyhedral oligomeric silsesquioxane (POSS) are a class of cage-shaped nanoscale building blocks that can self-assemble to construct hybrid organic/inorganic nanomaterials with enhanced physical properties[1-3], POSS nanocubes consist of silicon and oxygen atoms linked into a well-defined, cubic inorganic framework with silicon atoms at the corners and oxygen atoms interspersed along the edges. Each of the silicon corners can be functionalized with a variety of organic substituents[4,5] to confer precise control over the topology and architecture. Hu and Yuan[6,7] had proved that the octahedral silsesquioxane can be synthesized in the presence of Me4NOH. Scheme 1 illustrates the structural representation of the cubic silsesquioxane. In this paper, the hydrolyzation processing of the aliphatic aminosilsesquioxane was examined, and octaaminopropylsilsesquioxane (OAPS) was synthesized.
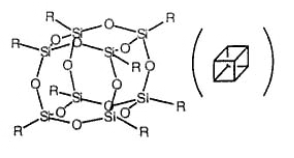
Scheme 1 The structural formula of the cubic silsesquioxane.
In cubic macromonomers, all eight vertices are functionalized and equally reactive. Upon processing, the size of the cube core and the functionalized arms define the size of the organic/inorganic components in the resulting nanocomposites where both components are distributed uniformly and periodically.
2. EXPERIMENTAL
2.1 Materials
g-aminopropyltriethoxysilane
(chromatographic degree of purity 98%), (CH3)4NOH·4H2O
was obtained from Beijing Fubide Fine Chemical Co. LTD. Methanol (99.5%) was obtained from
Tianjin Chemical Reagent Co. of China.
2.2 Preparation of OAPS
Octaaminopropylsilsesquioxane (OAPS) was prepared from H2NCH2CH2CH2Si(OCH2CH3)3
(0.25mol) and Me4NOH (0.77mol) under N2, the CH3OH
(500ml) was used as solvent. The reaction was first carried out at normal temperature for
24h, and then continued at 60ºC for 48h. The CH3OH was removed by
distillation, then the product was kept at 60ºC under vacuum for
another 48h. the leavings was kept at 110ºC for 24h to get rid of
superfluous Me4NOH and H2O. Finally, the product was purified by
100ml n-hexane and 100ml toluene, yield was 92.7%. The molecular weight was determined by
GPC and Vapor pressure osmometer (VPO).
2.3 Fourier transform IR spectra (FTIR)
FTIR was obtained using an Avatar series
Nicolet 205 FTIR spectrometer; optical grade potassium bromide (KBr) was used as a
background material. The resolution is ±2cm-1, and the spectral range is from 4800 to 200cm-1.
2.4 NMR Analyses
1H NMR analyses were performed with a Bruker DSX-600 spectrometer at 600MHz
with 10000Hz spectral width, a relaxation delay of 2s, a pulse width of 20ºC, and 30K data points. All the spectra were
recorded in D2O using the H2O signal as an internal reference.
13C NMR analyses were performed at 300MHz with 30KHz
spectral width, a relaxation delay of 5s, All the spectra were recorded in acetone medium.
Solid state 29Si NMR spectra were obtained at 300K using a
Bruker DSX-300 spectrometer operating at 59.6MHz. Pulse delays were 5s. The sample
temperature was 300K and TMS was used as reference.
2.5 Element analysis
Element analyses were performed with PE-2400.
2.6 Differential Scanning Calorimetry (DSC)
Calorimetry was performed on materials using a CDR-4P differential scanning calorimetry.
The airflow rate was 60ml/min. Sample (9-10 mg) was placed in a pan and raised to 400ºC
(10ºC/min/air) without capped. The heat flow differences between reference and sample
were recorded.
2.7 Melting point mensuration
X-4 microscopic melting point apparatus (Beijing) was used to observe the melting point of
Octaaminopropylsilsesquioxane (OAPS) at the heating rate of 2ºC/min.
3. RESULTS AND DISCUSSION
The process of formation of the silsesquioxane compounds is described as a multi-step
hydrolysis-condensation reaction (see Scheme 2). The first step is the hydrolysis of an g-amino- propyltrioethoxysiloxane with
water to form organic polysilanol compound. In the right environment, depending on the
concentration of water, solvent and pH, these precursors can condense with each other,
leading to the formation of oligosiloxanes. By means of thermo-dynamics[8,9],
kinetics and solubility of the products, the resulting product mixture can be
determined, ranging from lower oligosiloxane dimers or tetramers to polyhedral oligomeric
silsesquioxanes like the tri- and tetrasilanol silsesquioxanes.
The hydrolysis of
r-aminopropyltriethoxysilane in the presence of Me4NOH was studied with various
proportions. When the proportion is 3:1, crystal water of tetramethylammonium can provide
adequate H2O in hydrolyzation. The equal molar ratios of tetramethylammonium
and ethoxy can provide hydrolytic condition completely and then the reaction selectively
to form cube cage, while in other proportions the cage
cannot be formed or impurity product obtained. Less solvent could lead to ladder molecules
(Mn -20000), while more solvent decreased pH
value so that the cage could not be formed.
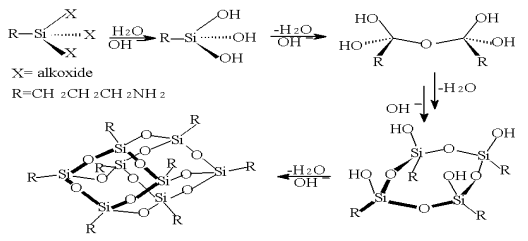
Scheme 2 The process of formation of the
silsesquioxane compounds
Molecular masses and distributions were measured by GPC and VPO as in Table 1. OAPS data are also shown as comparison. Because the hydrodynamic volumes of "spherical" OAPS are smaller than the linear polystyrenes used for GPC calibration, the measured molecular weights were smaller than the theoretical values. But the molecular mass is 810 determined by VPO, which is close to the theoretical value.
Table 1 GPC data of OAPS
GPC |
VPO |
||||
OAPS |
Calcda |
Mn |
Mw |
PDIb |
Mn |
880 |
714 |
1257 |
1.76 |
810 |
|
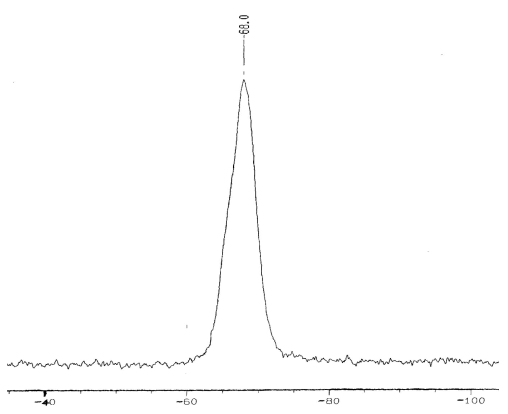
Fig.1 29Si NMR of OAPS
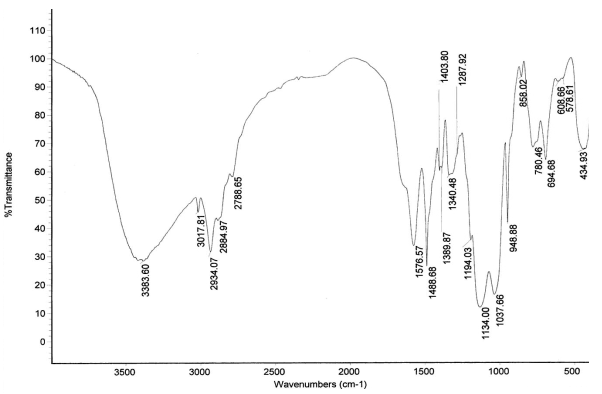
Fig.2 FTIR of OAPS
The analysis of FTIR is shown in Figure 2 and Table 2. Although vO-H band at 3300-3500cm-1 in the FTIR spectra is hardly distinguishable from the vN-H peak at ∽3300cm-1, 29Si NMR spectra (Fig. 1) clearly shows Si peak at -68.0 ppm, which suggests that 3383.60 cm-1 in the FTIR is vN-H peak (Fig. 2). The result of 29Si NMR spectra accord with the octaaminophenylsilsesquioxane characterized by Choi[10].
Table 2 FTIR, NMR and Element Analysis of OAPS
Composite |
Element analysis (%) |
FTIR |
NMR (ppm) |
||||
Theoretical value |
Measured value |
1 H |
13 C |
29 Si |
|||
[NH2(CH2)3SiO1.5]8 |
C |
32.727 |
32.855 |
Si-O-Si 1037.66-1134.00 C-N 1488.68 C-Si 948.88 C-C 1194.03 C-H 2934.07 C-H 1287.92-1340.48 C-H 1389.87-1403.80 |
NH2 1.521-1.607 gH 0.406- 0.476 long-chain alkyl 2.304- 2.475 |
aC 27.8 bC gC |
SiO2 |
H |
7.272 |
7.363 |
|||||
N |
12.727 |
12.851 |
|||||
O |
21.818 |
20.266 |
|||||
The correlative data can
be seen from Table 2, the theoretical value is close to the measured value in element
analysis. We can also obtain the conclusion that the molecular mass is close to
theoretical value. The ethoxyl groups have been hydrolyzed completely in the 1H
NMR and 13C NMR.
The thermal behaviors of OAPS have been characterized by means of DSC
(Fig.3) and melting point apparatus,OAPS melts at
196.6ºC and begins to decompose at 242ºC.
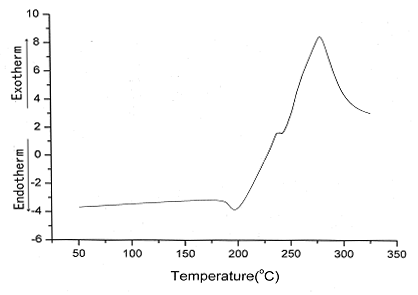
Fig.3 DSC of OAPS cube (10ºC/min/air,
first run)
4. CONCLUSIONS
When the proportion of tetramethylammonium and r-aminopropyltriethoxysilane is 3:1,
the OAPS can be synthesized in CH3OH. OAPS begins melting at 196.6ºC, which suggests it has high thermal stability.
REFERENCES
[1] Sanchez C, Ribot F. New J Chem, 1994, 18: 1007-1074.
[2] Lichtenhan J D. Comments on Inorganic Chemistry,
1995, 17: 115-130.
[3] Provatas A, Matisons J G. Trends in polymer science, 1997, 5: 327-332.
[4] Feher F J, Budzichowski T A. Polyhedron, 1995, 14: 3232-3253.
[5] Tamaki R M, Tamaki R, Choi J. WO 2002, A1: 100867.
[6] Yuan Changyou, Hu Chunye. Silicone Material, 2001, 15 (2): 1-4.
[7] Hu Chunye, Tanyan, Yuan Changyou. Chinese Science Bulletin, 1999, 17 (44): 1818-1820.
[8] Kudo T, Gordon M S. J. Phys. Chem. A 2000, 104: 4058-4063.
[9] Jug K, Wichmann D. J. Comp. Chem. 2000, 21: 1549-1553.
[10] Jiwon Choi, Seung Gyoo Kim, Richard M. Laine. Macromolecules 2004, 37: 99-109.
胺丙基倍半硅氧烷八聚体的合成与表征
高俊刚,张学建,王士臣,闰明涛
(河北大学化学与环境科学学院,保定,071002)
摘要 用(CH3)4NOH和胺丙基三乙氧基硅烷合成了八聚胺丙基倍半硅氧烷,对水解过程和反应条件进行了研究。用元素分析仪测定了各元素的含量。用GPC和VPO测定了产物的分子量,结果与理论值相符。用FTIR,
1H NMR, 13C NMR, 29Si NMR对产物的结构进行了表征。最后用DSC和显微熔点测量仪确定产物的热稳定性。
关键词 合成,八聚胺丙基倍半硅氧烷,表征