http://www.chemistrymag.org/cji/2005/077050pe.htm |
Jul. 2, 2005 Vol.7 No.7 P.50 Copyright |
Ultrasonic extraction method for the gas chromatographic determination of organochlorine pesticides residue in some herbs
Lu Ping, Song Baoan, Sui Wubin, Hu Deyu, Xue Wei, Jin
Linhong, Liu Gang, Huang Rongmao, Yang Song
(Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education,Research and Development Center for Fine Chemicals, Guizhou
University, Guiyang 550025 , China)
Received on Jun.17, 2005; Supported by the National Natural Science Foundation of China (No.20162001) and Natural Science Foundation of Guizhou Province (No.20043020) and Program for Excellent Talents of Science and Education of Guizhou Provincial Governor (No.200407).
Abstract An ultrasonic extraction and gas
chromatographic determination of organochlorine pesticides(OCPs) with electron captured
detector was described for a-BHC,
b-BHC, c-BHC, d-BHC, op' -DDT, pp' -DDT, pp' -DDE and pp' -DDD
of pesticide residues in Pinellia Tuber, Fleeceflower Root, Eucommiaulomoider Oliv,
Dendrobium, Medicinal Evodia Fruit and Lonicera japonica Thunb. Recoveries were varied
between 78-114% and relative standard deviations varied between 2.76% and 8.17%. The
detection and quantification limits for this method were 0.1 to 0.2 ng/g for the above six
pesticides.
Keywords Pesticide Residue; Herbs; ultrasonic fluid extraction; Gas Chromatography
1 INTRODUCTION
Chinese herbal medicines (CHMs) have been used by the Chinese for thousands of years.
They play an important role in Chinese daily life. Due to the increasing demand of the
CHMs, wild herb can hardly meet the requirements of the market because the wild resource
is limited. So many herbal plant are now being cultivated by manpower. During the herb
plant's cultivation, pesticides are widely used to control diseases and attacks originated
from insects and mites [1]. So there is possible risk for consumer to take
pesticide residues remaining on CHMs. The organochlorine pesticides (OCPs) are widely used
in the world. And their residual level has attracted the attention by scientists due to
their high accumulation, though many kinds of OCPs have been forbidden by many countries
throughout the world. Furthermore, the OCPs have a long half-life, harmful biological
effects on the environment [2].
The separation of OCPs in CHMs are always difficult at some problems
which are extraction, purification and detection of OCPs in complex matrices. Classical
extraction methods such as solvent extraction, column adsorption have been largely
replaced by solid-phase micro extraction (SPME) [3], supercritical fluid
extraction (SFE)[4]. However, although providing simple, fast methods, SPME
gives low extraction yields that are affected by the herb, and some pesticides are not
fully extracted[5]. SFE has been shown to be an efficient and rapid method for
the separation of pesticides from CHMs[6-8], but it needs special equipments.
We now report a simple and fast ultrasonic fluid extraction (UFE)
method for the determination of OCPs in CHMs, which served the benefits such as fast,
simple, lower cost, no special apparatus and only small amounts of solvents needed. And
the most important is that it has good reproducibility. All the recoveries varied between
78% to 111% and relative standard deviation (RSD) varied between 2.76% to 8.17%.
2 MATERIALS AND METHODS
2.1 Chemicals
All solvents used were of pesticide residue grade and anhydrous sodium sulfate was
analytical grade reagents (Shanghai Chemical Reagents Co.). The standard pesticide sample
of op' -DDT, pp' -DDT, a
-BHC, b -BHC, c -BHC, d -BHC, pp' -DDE, and pp' -DDD were purchased from the Institute of
Scientific Research of Environmental Protection, Ministry of Agriculture of the People's
Republic of China, which were certified as a concentration of 100mg/mL. Anhydrous sodium sulfate was
heated at 500ºC for 4h and was
stored at 50ºC.
2.2 Instrumentations
Chromatographic analysis were carried out using an Agilent 6890N gas chromatograph
equipped with a 63Ni electron captured detector (ECD) and a split-splitless
injector. Capillary column was a HP-5 (diphenyl(5%) dimethylpoly siloxane(95%) Quartz
capillary column 30m 0.32mm i.d., 0.25m m, Agilent, USA). Ultrasonic cleaner (KQ3200E).
2.3 Standard solutions
A stock standard solution at a concentration of 200ng/mL was prepared with petroleum
ether. Appropriate dilutions of this stock standard solution were made with petroleum
ether to obtain working standard solutions of a series of concentration of 1-200ng/mL.
2.4 Herb sample fortification
Herb samples were originated from Guizhou local plant field. They were fortified by adding
a combined standard of OCPs to obtain concentration of 20, 50, 200ng/g. After the
fortification the samples were kept at room temperature to equilibrate for 1 hr prior to
extraction. Five replicates at each fortification level, including unspiked controls, were
extracted, clear-up and analyzed by GC as described below.
2.5 Extraction
Herbs are ground mechanically to homogeneous powder and sieved through a No. 40 mesh
sieve. One gram of such powder was weighed into beaker and 5ml petroleum ether was added.
It was proceeded ultrasonic extraction for 15-20min. Then centrifuge for 5 min at high
speed. The upper layer was concentrated with a vacuum rotary evaporator equipped with a
water bath at 35ºC (Method for Pinellia Tuber and Dendrobium) or the
upper layer was injected into a tube and 0.1ml 95% concentrated sulfuric acid was added.
Then was proceeded ultrasonic extraction for 5min, centrifuging 3min and the upper layer
was added with 0.2g anhydrous sodium sulfate, ultrasonic extraction for 5 min, and
centrifuge 5 min. The upper layer was concentrated for analyses.
2.6 GC condition for 8 OCPs analysis
The inlet and detector were operated at 250ºC and 320ºC, respectively. Injection
mode is split, with split ratio 5:1. Oven temperature was intimately held at 60ºC for
1 min, increased to 160ºC (20ºC/min) and held for 5 minutes, increased to 260ºC
(10ºC/min) and held for 2 min.
Nitrogen was used as carrier and makeup gas at 100mL/min, 60 mL/min, respectively. Sample
injection volume was 1mL.
3 RESULTS AND DISCUSSION
The residue level of 8 OCPs in 6 Chinese herb samples were exhibited in Table 1, it
can be seen that the BHC and DDT residue level in Pinellia Tuber and Fleeceflower Root are
a little higher than the national standard.
Table 1 Contents of 8 OCPs in 6 Chinese herb samplesa)
pesticides |
Pinellia Tuber |
Fleeceflower Root |
Eucommiaulomoider Oliv |
Medicinal Evodia Fruit |
Lonicera japonica Thunb |
Dendrobium |
a -BHC |
16 |
16.9 |
NDb) |
7.9 |
10.8 |
14.0 |
b -BHC |
37.6 |
39.7 |
8.1 |
23.3 |
21.2 |
28.0 |
c -BHC |
21.5 |
23.5 |
8.0 |
9.0 |
38.3 |
18.0 |
d -BHC |
45.7 |
38.0 |
13.1 |
21.3 |
43.4 |
24.7 |
Total BHC |
120.8 |
118.1 |
29.2 |
61.5 |
113.7 |
84.7 |
pp¢ -DDT |
ND |
26.1 |
ND |
19.7 |
78.6 |
ND |
op¢ -DDT |
ND |
23.0 |
ND |
ND |
ND |
ND |
pp¢ -DDD |
57.1 |
58.3 |
5.9 |
17.0 |
15.7 |
37.0 |
pp¢ -DDE |
38.2 |
42.5 |
9.1 |
ND |
14.6 |
17.1 |
Total DDT |
149.7 |
149.9 |
15.0 |
36.7 |
108.9 |
54.1 |
a)
ng/g. b) ND means no detection3.1 Standard curve
A ECD detector response curve was obtained by injecting duplicate standard solutions
(1-200ng/mL). The response of 8 OCPs was linear in the range studied and the correlation
coefficient determined was 0.9987-0.9999, the detection and quantification limits for this
method were 0.1 to 0.2 ng (Table 2).
Table 2 The
linear regression equation for 8 OCPs
Pesticide |
Retention time/min |
Regression equation |
Correlative coefficient |
Linear range /g |
Detection limit /g |
a -BHC |
4.109 |
Y=2823.0X-89.5 |
0.9992 |
1.0×10-13-1.0×10-9 |
1.8×10-10 |
b -BHC |
4.406 |
Y=965.4X-6.4 |
0.9999 |
1.0×10-13-1.0×10-9 |
1.5×10-10 |
c -BHC |
4.531 |
Y=2098.4X-48.5 |
0.9990 |
1.0×10-13-1.0×10-9 |
1.2×10-10 |
d -BHC |
4.951 |
Y=1977.4X-44.3 |
0.9991 |
1.0×10-13-1.0×10-9 |
1.8×10-10 |
pp¢ -DDT |
9.483 |
Y=2033.2X-51.0 |
0.9996 |
1.0×10-13-1.0×10-9 |
1.4×10-10 |
op¢ -DDT |
10.730 |
Y=1088.6X+6.41 |
0.9998 |
2.0×10-12-1.0×10-9 |
1.9×10-10 |
pp¢ -DDD |
10.827 |
Y=955.8X-24.3 |
0.9997 |
1.0×10-13-1.0×10-9 |
2.1×10-10 |
pp¢ -DDE |
11.882 |
Y=925.7X-21.0 |
0.9987 |
2.0×10-12-1.0×10-9 |
1.5×10-10 |
3.2 Reproducibility
The inter-day reproducibility of the retention time and peak height were examined by using
a 10ng/mL standard and 50mg/g
spiked sample extracts throughout the course of the experiment. A total of 45 injections
of each over 15 days. The results showed that the Lonicerajaponica Thunb peak
height variabilities for both the 10mg/mL standard and the spiked herb extract were with 5.0%
R.S.D. Retention time fluctuation measured in the same way showed the maximum R.S.D value
of 2.7%. Gas chromatograms of the 8 OCPs standard samples were shown in Fig 1.
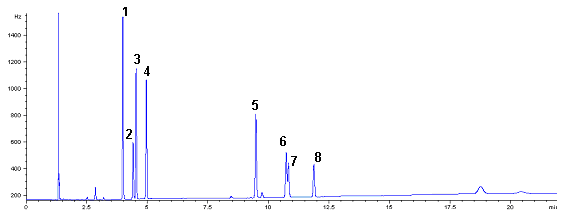
Fig 1 Chromatogram of 8 kind of OPPs standard
sample. 1, a -BHC; 2, b -BHC; 3, c-BHC; 4, d-BHC; 5, pp'-DDE; 6, pp'-DDD; 7, op' -DDT;
8, pp' -DDT
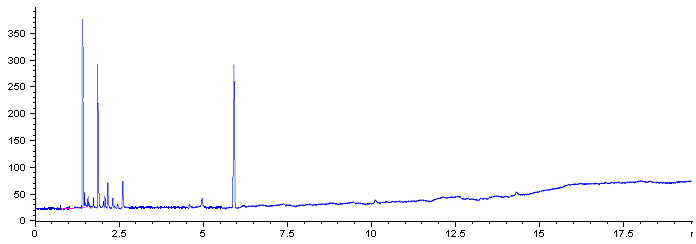
Fig 2A Chromatogram of organochlorine
pesticide of Matrix of Lonicerajaponica Thunb herb sample
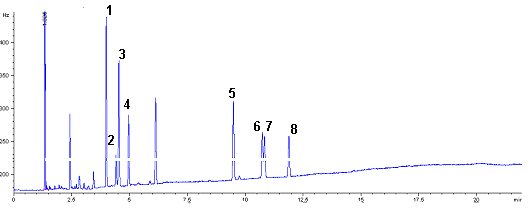
Fig 2B Chromatogram of organochlorine
pesticide of fortified Lonicerajaponica Thunb herb sample.
3.3 Recovery
Fig 2A and 2B shows GC chromatograms for unspiked herb and for herb spiked
at 100ng/g, respectively. The chromatograms for unspiked herb showed lack of interference
in the retention region for 8 OCPs. The recovery data for OCPs spiked into several herbs
are presented in Table 3. Mean recoveries of five replicates ranged from 80.7% to
104.6%, with relative standard deviation (RSD) values from 2.97% to 7.20%.
Table 3 Recoveries of 8 OCPs from
fortified herb samples at different concentration levels*
Pesticide |
Pinellia Tuber |
Eucommia ulmoidesOliv |
|||||||
Fortification levels |
Recoveries*(%) |
Fortification levels |
Recoveries*(%) |
||||||
Range |
Mean |
RSD |
Range |
Mean |
RSD |
||||
a -BHC |
20 |
89-97 |
92.6 |
4.10 |
20 |
81-86 |
83.7 |
3.35 |
|
50 |
92-98 |
94.6 |
3.07 |
50 |
81-91 |
86.1 |
5.46 |
||
200 |
92-98 |
95.1 |
3.18 |
200 |
89-97 |
93.7 |
4.06 |
||
b -BHC |
20 |
86-93 |
90.2 |
3.87 |
20 |
82-88 |
84.7 |
3.54 |
|
50 |
89-97 |
93.1 |
4.32 |
50 |
86-93 |
89.6 |
3.01 |
||
200 |
93-100 |
96.3 |
3.89 |
200 |
92-101 |
96.4 |
4.67 |
||
c -BHC |
20 |
85-94 |
89.7 |
4.44 |
20 |
82-94 |
87.9 |
6.37 |
|
50 |
88-95 |
91.6 |
3.56 |
50 |
87-99 |
93.4 |
6.21 |
||
200 |
91-99 |
94.7 |
4.29 |
200 |
98-109 |
102.4 |
6.54 |
||
d -BHC |
20 |
84-90 |
86.9 |
3.20 |
20 |
80-90 |
84.5 |
6.39 |
|
50 |
85-92 |
88.9 |
3.51 |
50 |
84-97 |
90.7 |
7.17 |
||
200 |
88-96 |
92.4 |
4.43 |
200 |
93-106 |
99.6 |
6.02 |
||
pp¢ -DDT |
20 |
85-91 |
87.9 |
3.17 |
20 |
84-99 |
91.6 |
7.73 |
|
50 |
89-97 |
92.6 |
4.23 |
50 |
92-103 |
97.8 |
5.57 |
||
200 |
92-100 |
96.4 |
4.27 |
200 |
97-114 |
105.3 |
7.71 |
||
op¢ -DDT |
20 |
84-93 |
88.6 |
4.85 |
20 |
85-96 |
90.8 |
6.01 |
|
50 |
89-97 |
93.5 |
4.26 |
50 |
91-104 |
97.1 |
6. 48 |
||
200 |
97-101 |
97.0 |
4.58 |
200 |
96-112 |
103.5 |
8.39 |
||
pp¢ -DDD |
20 |
84-88 |
85.9 |
2.76 |
20 |
83-91 |
87.4 |
4.46 |
|
50 |
86-92 |
89.1 |
3.39 |
50 |
87-100 |
93.5 |
6.56 |
||
200 |
88-96 |
92.0 |
3.99 |
200 |
95-104 |
99.7 |
4.88 |
||
pp¢ -DDE |
20 |
83-90 |
86.6 |
3.76 |
20 |
79-89 |
83.7 |
6.11 |
|
50 |
86-94 |
90.4 |
4.57 |
50 |
84-95 |
89.4 |
5.57 |
||
200 |
90-99 |
94.6 |
4.77 |
200 |
99-110 |
104.9 |
5.34 |
||
(*n=5)
Table 3 Recoveries of 8 OCPs from fortified herb samples at different
concentration levels*(Continued)
Pesticide |
Medicinal Evodia Fruit |
Fleaceflower Root |
|||||||
Fortification levels |
Recoveries*(%) |
Fortification levels |
Recoveries*(%) |
||||||
Range |
Mean |
RSD(%) |
Range |
Mean |
RSD(%) |
||||
a -BHC |
20 |
80-87 |
83.7 |
3.75 |
20 |
81-94 |
87.5 |
7.20 |
|
50 |
84-91 |
87.5 |
4.01 |
50 |
90-100 |
94.8 |
5.51 |
||
200 |
88-97 |
92.4 |
4.58 |
200 |
95-105 |
99.9 |
4.79 |
||
b -BHC |
20 |
80-87 |
82.9 |
4.54 |
20 |
78-83 |
80.9 |
5.07 |
|
50 |
83-92 |
87.5 |
5.35 |
50 |
82-91 |
86.7 |
5.33 |
||
200 |
88-99 |
93.3 |
5.91 |
200 |
88-98 |
92.8 |
5.86 |
||
c -BHC |
20 |
81-88 |
84.7 |
4.09 |
20 |
81-93 |
86.9 |
6.57 |
|
50 |
86-96 |
90.8 |
5.26 |
50 |
88-97 |
92.7 |
5.03 |
||
200 |
93-104 |
98.6 |
5.42 |
200 |
94-106 |
99.8 |
5.64 |
||
d -BHC |
20 |
80-93 |
86.3 |
7.15 |
20 |
80-84 |
81.7 |
4.65 |
|
50 |
88-101 |
94.5 |
7.13 |
50 |
82-95 |
88.4 |
8.17 |
||
200 |
97-111 |
104.6 |
6.52 |
200 |
91-104 |
97.5 |
6.97 |
||
pp¢ -DDT |
20 |
80-88 |
83.9 |
4.55 |
20 |
81-92 |
86.7 |
6.16 |
|
50 |
85-94 |
89.7 |
5.54 |
50 |
87-96 |
91.8 |
5.24 |
||
200 |
92-101 |
96.5 |
4.82 |
200 |
93-104 |
98.7 |
5.30 |
||
op¢ -DDT |
20 |
78-84 |
80.7 |
5.38 |
20 |
84-92 |
88.2 |
4.51 |
|
50 |
82-91 |
86.7 |
5.14 |
50 |
89-100 |
94.8 |
5.56 |
||
200 |
89-95 |
91.9 |
2.97 |
200 |
96-109 |
102.5 |
5.99 |
||
pp¢ -DDD |
20 |
84-92 |
87.9 |
4.45 |
20 |
78-84 |
80.9 |
3.19 |
|
50 |
90-100 |
94.8 |
4.83 |
50 |
84-90 |
86.7 |
3.77 |
||
200 |
97-105 |
100.7 |
3.84 |
200 |
88-98 |
92.8 |
5.06 |
||
pp¢ -DDE |
20 |
82-91 |
86.4 |
5.24 |
20 |
82-88 |
84.6 |
3.71 |
|
50 |
88-96 |
91.7 |
4.15 |
50 |
86-95 |
90.5 |
4.77 |
||
200 |
92-103 |
97.1 |
5.60 |
200 |
94-101 |
97.4 |
3.81 |
||
Table 3 Recoveries of 8 OCPs from fortified herb samples at different concentration levels (Continued)
Pesticide |
Dendrobium |
Lonicerd japonica Thunb |
|||||||
Fortification levels |
Recoveries*(%) |
Fortification levels |
Recoveries*(%) |
||||||
Range |
Mean |
RSD |
Range |
Mean |
RSD |
||||
a -BHC |
20 |
82-92 |
86.9 |
6.01 |
20 |
86-94 |
90.3 |
4.32 |
|
50 |
87-96 |
91.6 |
4.71 |
50 |
85-91 |
87.9 |
3.42 |
||
200 |
92-102 |
97.2 |
4.97 |
200 |
91-100 |
95.6 |
4.40 |
||
b -BHC |
20 |
83-93 |
88.3 |
5.55 |
20 |
89-94 |
91.6 |
3.20 |
|
50 |
88-97 |
92.4 |
4.69 |
50 |
96-106 |
101.3 |
4.73 |
||
200 |
91-101 |
96.1 |
5.34 |
200 |
90-100 |
95.2 |
5.35 |
||
c -BHC |
20 |
86-95 |
90.5 |
5.30 |
20 |
85-92 |
87.9 |
4.19 |
|
50 |
92-98 |
94.8 |
3.61 |
50 |
88-97 |
92.4 |
5.01 |
||
200 |
97-108 |
102.4 |
5.03 |
200 |
93-101 |
97.0 |
4.39 |
||
d -BHC |
20 |
88-98 |
92.6 |
5.52 |
20 |
88-96 |
92.1 |
4.38 |
|
50 |
94-102 |
97.3 |
4.81 |
50 |
82-93 |
87.5 |
5.82 |
||
200 |
96-106 |
101.2 |
5.03 |
200 |
89-99 |
93.7 |
5.30 |
||
pp¢ -DDT |
20 |
81-89 |
84.9 |
4.68 |
20 |
82-94 |
88.4 |
6.88 |
|
50 |
85-94 |
89.4 |
4.77 |
50 |
88-99 |
93.5 |
5.42 |
||
200 |
93-102 |
97.8 |
4.75 |
200 |
93-105 |
98.9 |
6.32 |
||
op¢ -DDT |
20 |
82-91 |
86.4 |
5.67 |
20 |
86-95 |
90.3 |
4.68 |
|
50 |
90-98 |
94.1 |
4.59 |
50 |
82-92 |
86.7 |
6.22 |
||
200 |
94-106 |
100.2 |
5.92 |
200 |
90-103 |
96.4 |
6.70 |
||
pp¢ -DDD |
20 |
80-86 |
82.8 |
4.55 |
20 |
81-88 |
84.8 |
3.97 |
|
50 |
83-93 |
88.6 |
5.11 |
50 |
87-97 |
92.1 |
5.19 |
||
200 |
90-97 |
93.9 |
4.16 |
200 |
98-109 |
103.5 |
5.54 |
||
pp¢ -DDE |
20 |
83-95 |
89.2 |
6.70 |
20 |
86-93 |
89.4 |
3.72 |
|
50 |
91-101 |
95.8 |
5.69 |
50 |
92-103 |
97.5 |
5.28 |
||
200 |
95-108 |
101.5 |
6.67 |
200 |
89-101 |
94.8 |
6.51 |
||
3.4 Extraction
In our study, ultrasonic extraction method was developed because of its good
efficiency. As for the solvent, petroleum ether were confirmed to be a good choice for the
OCPs considered its low polarity though the extraction solvent was changeable. Acetone and
ethyl acetate were also tested but more impurity were present in the extraction which made
it more difficult to purify the extraction.
Extraction time is another factor that would influence the extraction
efficiency and selectivity of the fluid. A decreased separation efficiency was observed
when extraction time decreased from 15min to 10min. It also could not be enhanced by
prolonging the extraction time which in the contrast, risk of contamination was increased.
Lower recoveries were got when the extraction time was shorter than 10 min and too long
time will increase the matrix interference. The extraction carried out at 30ºC would
be the best ( showed in Table 4).
Table 4 Optimized conditions and
comparison of methods related to OCPs detection in Lonicerajaponica Thunb
Entry |
Extraction solvent and method |
Extraction time |
Extraction temperature |
Decontamination rate a (%) |
1 |
petroleum ether and stirring |
8h |
Room temperature |
68.7 |
2 |
petroleum ether by UFE method |
15 |
25 |
76.9 |
3 |
Acetone + petroleum ether (2+1) with UFE method |
15 |
25 |
70.5 |
4 |
petroleum ether by UFE method |
10 |
30 |
87.5 |
5 |
Acetone + petroleum ether (2+1) with UFE method |
15 |
30 |
78.1 |
6 |
petroleum ether by SFE method |
15 |
30 |
88.0 |
4 CONCLUSION
The method described in this paper
allows the determination of 8 OCPs residues at one time in 6 CHMs which are Pinellia
Tuben, Fleeceflower Root, Eucommiaulomoider Oliv, Dendrobium, Medicinal Evodia Fruit and
Lonicera japonica Thunb. The precision and accuracy of the method were encouraging.
The advantages of this method such as rapid, relatively simple, small amount of solvents
needed and only small sample sizes required make this method particularly attractive in
pesticide residue analyzing.
Abbreviations used: UFE, ultrasonic fluid extraction; OCPs, organochlorine pesticides; op' -DDT, 1,1,1-trichloro-2-(2-chlorophenyl)-2-(4-chlorophenyl)-ethane; pp' -DDT, 1,1,1- trichloro-2,2-bis(4-chlorophenyl)-ethane; a-BHC, a -hexachlorocyclohexane, b -BHC, b - hexachlorocyclohexane,c -BHC, c -hexachlorocyclohexane, d-BHC, d-hexachlorocyclohex- ane, pp' -DDE, 1,1-dichloro-2,2-bis (4-chlorophenyl)-ethylene; and pp'-DDD, 1,1-dichloro -2,2-bis(4-chlorophenyl) ethane.
REFERENCES
[1] R.S. Sheridan, J.R. Meola, J. AOAC Int. 1999, 82: 982.
[2] Y. Cai, C. Zhao, Z. Guan, G. Chen, L. Wang, J. Shengyang Pharmaceut. Univ.
1997, 14: 283.
[3] B.H Hung, H. Wang, M.R.Lee, J. . Chromaogr. A 2000, 898:245.
[4] C.J. Zhao, G.M. Hao, H.X. Li, Y.G. Chen, Biomed. Chromat. 2002, 16: 441.
[5] M. Volante, M. Pontello, L. Valoti, M. Cattaneo, M. Bianchi, L.Colzani, Pest Manag.
Sci. 2000, 56: 618.
[6] L.J. Barnabas, J.R. Dean, S.M. Hitchen, J. Chromatogr. Sci. 1994, 32: 547.
[7] C. Nerin, R. Batlle, J. Cacho, J. Chromatogr. A. 1998, 795: 117.
[8] J. Li, F. Ge, X. Huang, J. Chin. Med. Mater. 1996, 19: 187.
超声提取法用于气相色谱检测几种中草药中有机氯农药残留
卢平 宋宝安 隋吴彬 胡德禹 薛伟
金林红 刘刚 黄荣茂 杨松
(教育部绿色农药与农业生物工程重点实验室,贵州大学精细化工研究开发中心
贵阳 550025)
摘要 超声提取法和气相色谱(63Ni电子捕获检测器)法用于测定半夏、何首乌、杜仲、石斛、吴茱萸、金银花等中药材中的a-BHC, b-BHC, c-BHC, d-BHC, op'-DDT, pp'-DDT, pp'-DDE 和 pp'-DDD等有机氯农药残留含量。添加回收率在78%-114%之间,相对标准变异系数2.76%-8.17%。该方法对六种中草药的检测限为0.1-0.2
ng/g。
关键词 农药残留;中草药;气相色谱方法