http://www.chemistrymag.org/cji/2005/077052pe.htm |
Jul. 22, 2005 Vol.7 No.7 P.52 Copyright |
(College of Chemistry &Chemical Engineering, Northwest Normal University, Lanzhou 730070, China)
Received on Apr.30, 2005. Abstract A new chemical oscillating reaction system involving the manganese (II)-catalyzed reaction among potassium bromate, acetone and tyrosine in acidic medium was described in this paper. The apparent activation energy of the induction period (Ein) and that of oscillation period (Ep) were calculated, Ein=110.61KJ/mol, Ep=159.41KJ/mol. The optimum concentration of each reactant was investigated and a steady chemical oscillating system was obtained. The influence of temperature was studied as well. The mechanism of the reaction was also explored in detail and a possible quantitative explanation was proposed. 1 INTRODUCTION
Since the first paper [1] about chemical oscillating reaction was published, the chemical oscillating reactions have attracted the attention of researchers for many years because of their challenging chemistry and their wealth of dynamic behavior [2-5]. Part of the interest in chemical oscillating reaction grew from the study of non-linear dynamics applied to physics [6] and biology [7, 8], even meteorology [6] and economic [9]. The connection between the life process that exhibits oscillating behavior and chemical oscillating system is also one of the most interesting study subjects. An important, unifying discovery is that many complex natural phenomena are governed by simple dynamic laws. Chemical oscillating reactions are no exception, because they exhibit complex behavior often based on relatively simple dynamic rules.
In a well-stirred chemical oscillator, the concentrations of certain intermediates vary over time, such a system must be thermodynamically far from equilibrium with an overall ¡÷G which is large and negative. In addition, the rate laws for the reaction must be non-linear, that is, some of the terms must be overall second order, or higher in the concentration of intermediates. Instabilities are usually related to autocatalysis, self-inhibition or delayed feedback. However, it is also important to note that the concentrations of the major reactants in chemical oscillator decrease slowly and continuously during the reaction process without an influx of one or more reagents to sustain oscillating. In consequence, the reaction continuously flows in the direction of decreasing free energy: there is no oscillation in the direction of the overall reaction, which is always moving inexorably towards the chemical equilibrium state.
Studies of chemical oscillating reactions are in two major aspects: (a) Application of regular chemical oscillation to analytical determination based on the relationship between the change caused by the analyte in the characters (amplitude, period and so on) of chemical oscillation system and the concentrations of the analyte. (b) Designing new chemical oscillating reactions and elucidating the intricate underlying mechanisms of them from the physico-chemical point of view.
It has been found that in the Belousov-Zhabotinskii (B-Z) system the catalyst cerium (IV) can be replaced by manganese (II), Fe(phen)2+, Ru(bipy)2+, tetraazamacrocyclic copper (II) or nickel (II) complexes [10, 11], and the organic substrate citric acid can also be substituted with malic acid, pyruric acid, eaffic acid and so on [12-15]. Until now, the chemical oscillator with amino acids as the substrate has not been reported. In the survey of the chemical oscillating behavior of amino acids in place of malonic acid in the BZ reaction in the presence of manganese (II) as the catalyst, we found that tyrosine can give rise to the chemical oscillating in the presence of acetone with the proper concentrations of the reactants. At the optimal oscillating conditions, the oscillating phenomenon can last more than one hour. 2 EXPERIMENT
2.1 Chemical
All chemicals were of analytical-reagent grade and were used as received. Solutions of 0.2 mol/L potassium bromate, 0.02 mol/L tyrosine and 0.2 mol/L manganese sulphate were prepared in 1.0 mol/L sulfuric acid respectively. Solution of 0.2 mol/L acetone was made daily. Doubly distilled deionized water was used throughout.
2.2. Apparatus
The instrumental consisted of a 50 ml glass reaction vessel fitted with a Model CS-501 thermostat (Shanghai Pujiang Analytical Instrumental Factory) and a magnetic stirrer Model ML-902 (Shanghai Pujiang Analytical Instrumental Factory) for homogenization. The experiments were performed with a CHI 832 electrochemical analytical system (Shanghai Chenhua Instrumental Company) in a conventional three-electrode cell to record the potential change. A Type 213 platinum electrode or a Type 302 bromide selective electrode was used as working electrode. A Type 213 platinum electrode as the counter electrode and a Type 217 saturated calomel electrode as the reference electrode against which all potentials were reported.
2.3. Procedure
2.3.1. Potentiometric measurements
Potentiometric measurements were performed in a closed thermostat-regulated glass container equipped with a magnetic stirrer. The stirring rate was kept at 500 rpm. In order to obtain stable oscillating system, different concentrations of reactants were mixed in the glass container and the potential was recorded by electrochemical analytical instrument equipped with a computer. The potassium bromate was added just before the record began. 3 RESULTS AND DISCUSSION
3.1 Results
The proposed chemical oscillating system exhibits periodic change both in the potential and in the color of the solution. The color of the solution is light yellow at the minimum of the cycle and brown at the maximum of the cycle. Fig.1 shows the typical oscillating profile for the new oscillating chemical system. Fig.1A recorded the simultaneous potential of a platinum electrode. This electrode measured a mixed potential affected by concentrations of manganese as well as bromine ion. Fig.1B recorded the change of bromide ion concentration with a bromide-selective electrode.
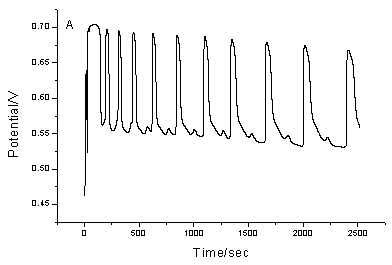
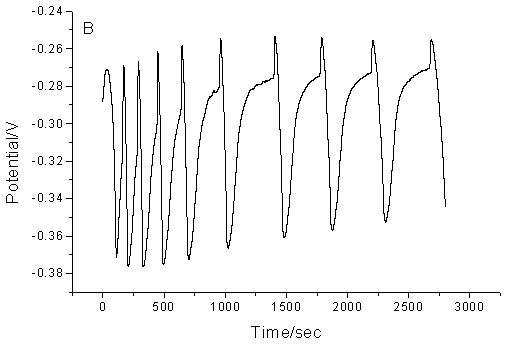 Fig.1 The typical
oscillation profile for the proposed oscillating system. (A), platinum electrode as
working electrode; (B), bromide ion-selective electrode as working electrode. Common
conditions: Tyrosine, 0.06 mol/L; KBrO3, 0.06 mol/L, CH3COCH3,
0.06 mol/L; Mn(II), 8.70¡Á10-3 mol/L; H2SO4: 1.26.mol.L-1
The periodicity in the rate of heat evolution during
oscillating in some autocatalytic systems has been described by Körös
et al [16]. We also studied the influence of temperature on the proposed
chemical oscillator. The results show that the induction period (tin), the time
from reactant mixture to appearance of regular oscillation, and oscillation period (tp),
the time of an integrated oscillation, depend greatly on temperature. Raising the
temperature made both the induction period (tin) and oscillation period (tp)
dramatically decrease whereas the oscillation amplitude remained almost constant (Fig.2).
In addition, ln(1/ tin) and ln(1/ tp) are linearly proportional to
the 1/T (Fig.3).
Fig.1 The typical
oscillation profile for the proposed oscillating system. (A), platinum electrode as
working electrode; (B), bromide ion-selective electrode as working electrode. Common
conditions: Tyrosine, 0.06 mol/L; KBrO3, 0.06 mol/L, CH3COCH3,
0.06 mol/L; Mn(II), 8.70¡Á10-3 mol/L; H2SO4: 1.26.mol.L-1
The periodicity in the rate of heat evolution during
oscillating in some autocatalytic systems has been described by Körös
et al [16]. We also studied the influence of temperature on the proposed
chemical oscillator. The results show that the induction period (tin), the time
from reactant mixture to appearance of regular oscillation, and oscillation period (tp),
the time of an integrated oscillation, depend greatly on temperature. Raising the
temperature made both the induction period (tin) and oscillation period (tp)
dramatically decrease whereas the oscillation amplitude remained almost constant (Fig.2).
In addition, ln(1/ tin) and ln(1/ tp) are linearly proportional to
the 1/T (Fig.3).ln(1/ tin)= 13.30391(1/T)£5.53698 (r=0.9981)
ln(1/ tp)= 19.17343(1/T)£7.03472 (r=0.9976)
Compared the above described equations with the Arrhenius equation:
lnk=£Ea/RT+A
The apparent activation energy (Ein) of the induction period and that of the oscillation period (Ep) was calculated, Ein=110.61KJ/mol, Ep=159.41KJ/mol. Although they could not explain the difficulty degree of each elementary reaction, because of Ein is less than Ep, they could illustrate that the occurrence of induction reaction is easier than that of oscillation reaction.
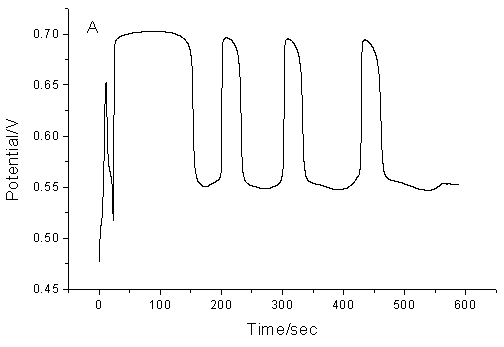
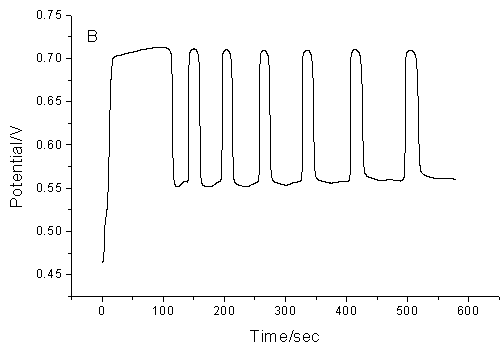
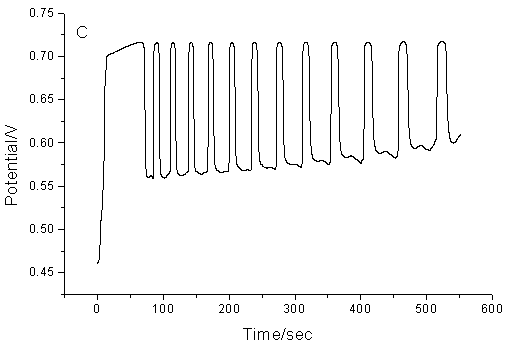 Fig.2 The
influence of temperature on the proposed chemical oscillation system. (A), 303.2K; (B),
313.2K; (C), 323.2K (Other conditions are the same as that in Fig.1)
Fig.2 The
influence of temperature on the proposed chemical oscillation system. (A), 303.2K; (B),
313.2K; (C), 323.2K (Other conditions are the same as that in Fig.1)
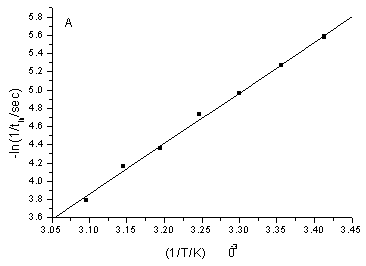
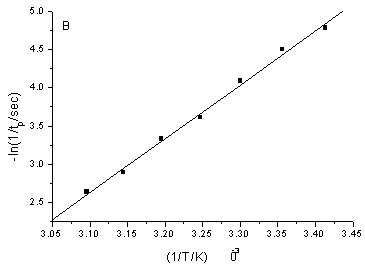 Fig.3
Calibration curve of the negative logarithm of the induction period (tin) or
the oscillation period (tp) versus the reciprocal of time (1/T/K). T from
293.0K to 323.0K, step is 5K. The oscillation conditions are the same as that in the
Fig.1.
3.2 Effects of
reaction components on the oscillator
Fig.3
Calibration curve of the negative logarithm of the induction period (tin) or
the oscillation period (tp) versus the reciprocal of time (1/T/K). T from
293.0K to 323.0K, step is 5K. The oscillation conditions are the same as that in the
Fig.1.
3.2 Effects of
reaction components on the oscillatorTo obtain stable oscillating system, the effects of the concentrations of tyrosine, potassium bromate, acetone, manganese (II) and sulfuric acid were examined.
The effect of tyrosine on the oscillating was investigated over the range from 2.0¡Á10-4 to 0.2 mol/L. As the initial concentration of tyrosine was lower than 4.35¡Á10-3 mol/L in the system, the system has no oscillating phenomenon, and the potential was low. If some more tyrosine was added into this system at this time, the potential increased immediately, and then the system began to oscillate. The lower the concentration of tyrosine, the shorter the duration of the system. This phenomenon demonstrated that tyrosine was consumed in the process of oscillating. However, the higher concentration of tyrosine may make the duration of oscillating shorter. So an initial concentration of 0.06 mol/L was finally adopted.
The effect of acetone was investigated over the range from 0.017 to 0.13 mol/L. If acetone was replaced by equal volume water, the color of solution was light yellow and the system could not oscillate. When the concentration of acetone was decreased, the duration decreased too. So the function of acetone is to remove surplus bromine that was produced in the induction period. Nevertheless, the more acetone was introduced into the system, the larger background noise was obtained. So 0.06 mol/L acetone was chosen as optimal.
The effect of potassium bromate in the system was also studied. The results showed that when the concentration of potassium bromate was lower than 0.038 mol/L, the same phenomenon was observed just as that of tyrosine. This observation illustrated that lower concentration of potassium bromate led to the concentration of Br- so low to be under the critical value that the system could not oscillate. The concentration of potassium bromate was higher; the color of solution in the induction period was deeper. However, when the concentration of potassium bromate was higher than 0.10 mol/L, the oscillating system is unsteady. We finally chose to employ 0.06 mol/L potassium bromate in the system.
The results of studying the influence of manganese (II) showed that potassium bromate cannot oxidize tyrosine without manganese as catalyst, but surplus manganese (II) in the system might have consumed certain crucial intermediate and lead to the disappearance of oscillating. In the proposed system the concentration of manganese (II) was chosen as 8.70¡Á10-3 mol/L.
The effect of sulfuric acid was different from that of tyrosine, acetone, potassium bromate and manganese (II). When the concentration of sulfuric acid was lower than 1.15 mol/L, the potential decreased at the minimum of the cycle (Fig. 4). With the concentration of sulfuric acid increasing, the change in potential (¡÷A) minished. However, too high concentration of sulfuric acid leads to an unsteady system. So 1.26 mol/L sulfuric acid was adopted as the optimal.
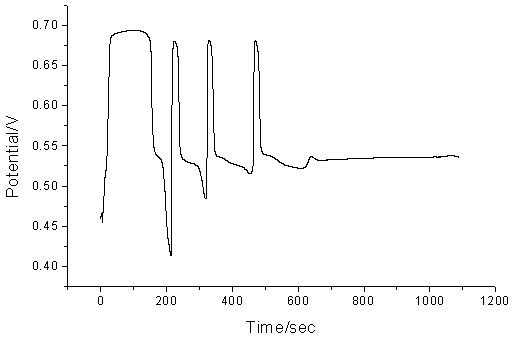
Fig.4 The typical oscillation profile for the proposed oscillating system with the low concentration of sulfuric acid. Conditions: Tyrosine, 0.06 mol/L; KBrO3, 0.06 mol/L, CH3COCH3, 0.06 mol/L; Mn(II), 8.70¡Á10-3 mol/L; H2SO4: 1.00 mol/L. Fig.5 has been drawn to illustrate the behavior of the electrodes, but the oscillation continued with only gradual damping. Both the trace of bromide-selective electrode and that of platinum electrode in Fig.5 can be clearly divided into five different periods that are associated with the oscillation itself. In the AB period, manganese (II) consumes slowly while bromide ion consumes rapidly. In the BC period, the change of bromide ion concentration is just as that in AB period whereas manganese (II) consumes rapidly. In the CD period, manganese (II) products consume slowly, but bromide ion products moderately rapid. In the DE period, both manganese (II) and bromide produce consume slowly. Finally, very steep EF period is designated as rapid manganese (II) and bromide ion production period. Then both the platinum and bromide-selective electrodes repeat the above described behaviors. With the reaction proceeding, the period of manganese (II) and bromide regeneration prolongs, so the period of oscillation prolongs too.
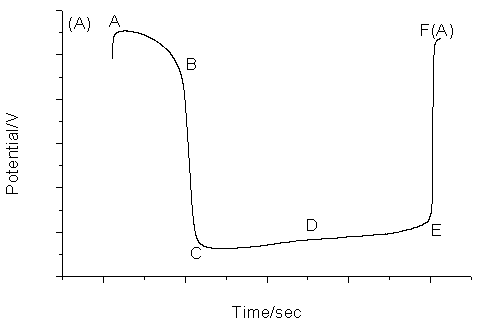
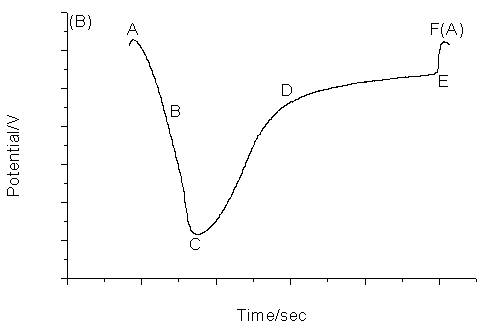
Fig.5 The behavior of platinum electrode as working electrode (A) and bromide ion-selective electrode as working electrode (B). 3.3 Mechanism
In general, the well-known FKN mechanism [17] can be applied to elucidate the basic mechanism of bromate ion-driven chemical oscillation reaction. According to this interpretation, the proposed system might involve three processes: Process A, Process B and Process C.
Process A:
BrO3-+4Mn2++5H+
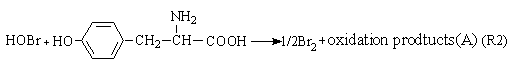
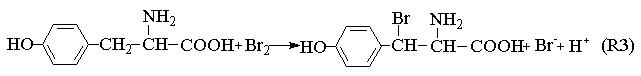
BrO3-+6H++Br-
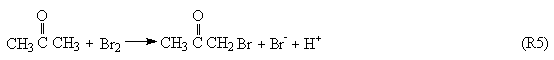
Process B:
BrO3-+Br-+2H+
HBrO2+Br-+H+
HBrO+Br-+H+
BrO3-+HBrO2+H+
BrO2·+Mn3++ H2O
Process C:
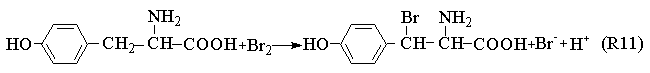
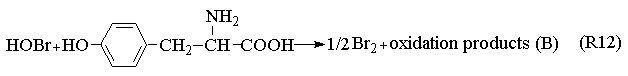
BrO2·+Mn2++H+
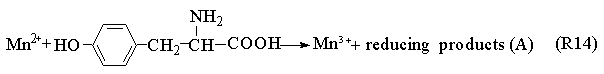
The Process A indicates the reactions in the induction period. When potassium bromide was introduced into the system, the R1 carry through in no time. Because of the bromine production, the color of the solution is yellow. Enolizated acetone in acidic media removed the surplus bromine in the system. With the development of the reactions, the color of the solution becomes light, and the concentration of bromide ion increases. When Process A has drived [Br-] to overrun a certain critical value that Process B happened and the system begins to oscillate. Bromide ion is consumed in the Process B. Process C begins when the concentration of bromide ion too low to maintain the Process B carrying out normally, the system is back to in the control of Process B, and then the system repeats the above-described behavior until the concentration of one of the reactants falls below the level necessary to sustain the cycle.
It was difficult for obtaining an exact mechanism because of the lack of some relevant thermodynamic data. However, computer simulation may be useful for solving the oscillation problem and the corresponding studies are in progress. Acknowledgment This work is sponsored by the Project of Youth Teachers Foundation of Education Ministry, China (3076), the Project of KJCXGC-01, NWNU, China, and National Natural Science Foundation of China (29875018). REFERENCES
[1] Belousov B P. Ref Radiats Med, 1958:145.
[2] Geest T, Olsen LF, Steinmets CG, Larter R, Shafer WM. J Phys Chem, 1993, 97:8431.
[3] Strizhak PE, Didenko OZ, Ivashchenko TS. Anal Chim Acta, 2001, 428:15.
[4] Yoshihiro Sasaki. Bull Chem Soc Jpn, 1999, 72:2607.
[5] Yatsimirskii KB, Strizhak PE, Ivaschenko S. Talanta, 1993, 40:1227.
[6] Li R. Non-equilibrium thermodynamic and dissipation structure theory, Qinhua Daxue Press, Beijing,1986.
[7] Olson DL, Scheeline A. Anal Chim Acta, 1990, 237:381.
[8] Jimenez-Prieto R, Silva M, Perez-Bendito D. Analyst, 1996, 121:563.
[9] Gleich J, Chaos: Making a new science, Penguin, New York, 1987.
[10] Xu J D, Ni S S, Inorg Chem, 1986, 25:1246.
[11] Xu Z Q, Xu J D, Ni S S. Acta Sinica Chim,1992, 50:734.
[12] Rastogi R P, Rastogi P, Kumar A, Indian J Chem,1977, 15A:163.
[13] Zhang Y J, Xu ZQ, Zhao L. Chin Chem Lett, 1994, 5:295.
[14] Chu L, Jiang P C, Xu HH. Acta. Chimica Sinica, 1991, 49:763.
[15] An C, Zhuang L, Liu Y, Lin Z. Acta Chemica Sinica, 1997, 55:259.
[16] Körös E, Orban M, Nagy Z. J Phys Chem, 1973, 77:3122.
[17] Field R J, Körös E, Noyes R M. J Am Chem Soc, 1972, 94:8649. ¡¡