http://www.chemistrymag.org/cji/2005/079058pe.htm |
Sep. 22,
2005 Vol.7 No.9 P.58 Copyright |
Wu Xinguo1, Cai Ruxiu2
and Lin Zhixin2
(1School of Resources and
Environmental Science, Wuhan University, Wuhan 430079, China; 2School of
Chemistry and Molecular Science, Wuhan University, Wuhan 430072, China)
Received on Jun.27, 2005; Supported by National Science Foundation of China (Grant No 20377032).
Abstract This paper describes an
extensive kinetic study of the oxidizing reactions of epinephrine and norepinephrine with
various oxidizers. It is concluded that the reactions of the two catecholamines with
different oxidizers can be characterized as successive reactions, and different
intermediate products and final products are formed under different oxidizers, pH values
and other experiment conditions. An oxidizing pathway is proposed to interpret the
experiment results. A quantitative prediction of the kinetic behavior of the oxidizing
reaction for the two catecholamines is well established by using successive kinetic
models.
Keywords oxidation pathway, successive reaction, Epinephrine, Norepinephrine.
Epinephrine (EP) and Norepinephrine (NE) play
important roles as neurotransmitters or hormones in the maintenance of homeostasis. The
major metabolism route of these cateholamines are O-methylation and oxidative deamination,
but there still remains a minor pathway in which they are oxidized to different stages
until melanin is formed[1]. What are the intermediate products still remains
unclear. Kinetic study on the reaction of EP with H2O2, which
catalyzed by Cu(II), reveals that two consecutive steps are involved[2,3]:
Adrenaline → Adrenochrome →
other products
ESR study reveals that free radical species are formed during enzymatic
oxidation, and it may be a reason for heart disease[4].
This paper present the results of a detailed kinetic study of the
reaction of EP and NE with different oxidizers, such as tris(1,10-phenanthroline)iron(III)
complex [Fe(phen)33+], Potasium hexacyanoferrate(III) [K3Fe(CN)6],
Oxygen (O2), and Horsradish Peroxydase/Hydrogen Peroxide system (HRP/H2O2)
etc.. The reactions are generally found to be successive reactions and the intermediate
products varied with different experiment conditions. An oxidation pathway is proposed for
EP and NE based on the experiment results. A mathematical model based on successive
reactions describes the kinetic behavior of these systems very well.
1 EXPERIMENTAL
1.1 Instrumentation
The flow-injection-stopped-flow (FISF) instrument used for kinetic studies consisted of a
UVIKON 941 spectrophotometer or a SFM25 spectrofluorimeter (Kontron Instrument, Zurich,
Switzerland) and a masterflex peristaltic pump (Cole-Parmer Instrument, Chicago, IL, USA).
The flow cell and sampling system were kept at a constant temperature by circulating water
from a model TB-85 thermo bath (Shimadzu, Kyoto, Japan). The fluorescence versus time
graph was recorded with a model 3056 pen recorder (Sichuan 4th Instruments Factory,
Sichuan, China). The absorbances versus time data were collected on-line by UVIKON 941.
Kinetic data were then transferred to an IBM compatible computer for processing with
programmes written in VB6.0 language by the authors. Multipoint curve-fitting methods were
used to process the signal vs. time data [5].
1.2 Reagents
Unless otherwise stated, all chemicals used were analytical-reagent grade and all
water used was doubly distilled from quartz vessels.
Stock solutions of 4.545×10-3mol/L epinephrine
hydrochloride and 6.250×10-3mol/L norepinephrine bitartrate were prepared in 1×10-2mol/L
hydrochloric acid by using the two catecholamine standards (Test Institute of Medicine and
Bioproducts, Health Ministry, China), and stored in a refrigerator. Working standard
solutions were prepared fresh daily by diluting the stock solution with 1×10-2mol/L
hydrochloric acid.
5×10-3mol/L Fe(phen)![]() were prepared before use by mixing 5ml 0.1mol/L NH4FeSO4
solution and 5ml 0.32mol/L phen solution in 100ml calibrated flask and diluting to volume
with 1.5mol/L acetate buffer of desired pH. Other concentration of Fe(phen)
were prepared before use by mixing 5ml 0.1mol/L NH4FeSO4
solution and 5ml 0.32mol/L phen solution in 100ml calibrated flask and diluting to volume
with 1.5mol/L acetate buffer of desired pH. Other concentration of Fe(phen)![]() in different pH are prepared similar as above.
in different pH are prepared similar as above.
0.08 mol/L K3Fe(CN)6 solution were prepared
before use by diluting 1 mol/L K3Fe(CN)6 with acetate buffer(pH
4.5).
1.0 mg·mL-1 HRP solution was prepared by dissolving
10.0mg HRP crystals(RZ∽3.0, activity>250m·mg-1, Dongfeng
Technology Company, Institute of Shanghai Biochemistry, Academy of P.R.China) in 10mL
water, and stored in a refrigerator.
5.6×10-3mol·L-1H2O2
was prepared by diluting 30% H2O2 with water (concentration was
estimated according to e =43.6 L· mol-1·cm-1 at 240nm)
0.1mol· L-1 phosphate buffer (pH 7.4) was prepared by dissolving 13.6g
KH2PO4 and 3.2g NaOH in 1 L calibrated flask, adjusting the pH to
7.4, as measured by a pH meter, and diluting to volume with water.
1.3 Procedure
For studies on the reaction of EP and NE with Fe(phen)![]() , UVIKON941 was set in TIME DRIVE mode, detection wavelength was set at
510nm, measurement time was set at 3 min, sampling rate was set at 100 samples/min,
thermobath was controlled at 45ºC. Suitable amounts of reagent and sample were placed in two tubes
housed in the thermobath. After the system had reached the desired temperature, reagent
and sample were sucked into mixing region from two streams by turn on the pump, and then
stopped in the cell by turn off the pump. The kinetic curves were recorded and saved by
UVIKON941. Twenty data points covering the whole kinetic curve were entered into the
computer for calculation.
, UVIKON941 was set in TIME DRIVE mode, detection wavelength was set at
510nm, measurement time was set at 3 min, sampling rate was set at 100 samples/min,
thermobath was controlled at 45ºC. Suitable amounts of reagent and sample were placed in two tubes
housed in the thermobath. After the system had reached the desired temperature, reagent
and sample were sucked into mixing region from two streams by turn on the pump, and then
stopped in the cell by turn off the pump. The kinetic curves were recorded and saved by
UVIKON941. Twenty data points covering the whole kinetic curve were entered into the
computer for calculation.
For studies on the reaction of EP and NE with Fe (CN )63-,
the procedure was similar as described above, except that temperature was controlled at 40ºC and detection
wavelength was set at 490 nm.
For studies on the reaction of EP and NE with dissolved O2
in alkaline solution, -, the procedure was similar as described above, except
that the variation of fluorescence intensity with time was monitored at lex = 410 nm and lem = 510 nm using the
spectrofluorimeter and recorded by the pen recorder.
For studies on the reaction of EP and NE with H2O2/HRP
system, the procedure was similar as with Fe(phen)![]() , except that detection wavelength was set at 490nm, measurement time was
set at 10 min, sampling rate was set at 50 samples/min, thermobath was controlled at 25ºC.
, except that detection wavelength was set at 490nm, measurement time was
set at 10 min, sampling rate was set at 50 samples/min, thermobath was controlled at 25ºC.
2 RESULTS AND DISCUSSION
2.1 Reaction with Fe(phen)![]()
Epinephrine or norepinephrine was found to react with ferroin complex Fe(phen)![]() in weakly acidic medium to form the red ferroin
Fe(phen)32+complex, which yields an absorption peak at 510 nm. These
reactions are quite rapid, but using the FISF technique, they can be followed by
monitoring the reaction rate spectrophotometriclly at the absorption wavelength of the
ferroin formed. Fig.1 shows the absorbance versus time graphs obtained for EP and NE on
reaction with Fe(phen)
in weakly acidic medium to form the red ferroin
Fe(phen)32+complex, which yields an absorption peak at 510 nm. These
reactions are quite rapid, but using the FISF technique, they can be followed by
monitoring the reaction rate spectrophotometriclly at the absorption wavelength of the
ferroin formed. Fig.1 shows the absorbance versus time graphs obtained for EP and NE on
reaction with Fe(phen)![]() in different pH values
by using the FISF technique for mixing sample and reagents. As can be seen, the variation
of pH values has changed not only the rate constants of the reactions, but also the
sensitivity of the absorbance. Because the molar absorptivity of ferroin is constant in a
wide range of pH and is about 1.15 × 104 L mol-1cm-1,
the changes in sensitivity must refers to that different amount of ferroin has been
formed. Another feature is that several kinetic curves of both EP and NE, obtained at
different pH values, are obviously divided into two parts, the starting phase are fast and
the terminal phase are slow. All these observations make us realize that the reaction of
EP and NE with Fe(phen)
in different pH values
by using the FISF technique for mixing sample and reagents. As can be seen, the variation
of pH values has changed not only the rate constants of the reactions, but also the
sensitivity of the absorbance. Because the molar absorptivity of ferroin is constant in a
wide range of pH and is about 1.15 × 104 L mol-1cm-1,
the changes in sensitivity must refers to that different amount of ferroin has been
formed. Another feature is that several kinetic curves of both EP and NE, obtained at
different pH values, are obviously divided into two parts, the starting phase are fast and
the terminal phase are slow. All these observations make us realize that the reaction of
EP and NE with Fe(phen)![]() are successive
reactions, and at different pH values, the intermediate product and final product are
different as regard to the electrons lost or the numbers of ferroin molecules formed per
molecule of EP or NE.
are successive
reactions, and at different pH values, the intermediate product and final product are
different as regard to the electrons lost or the numbers of ferroin molecules formed per
molecule of EP or NE.
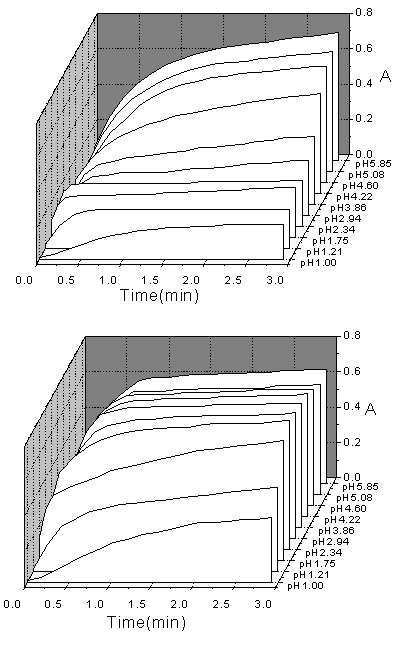
Fig.1 Kinetic curve of EP and NE on the reaction with Fe(phen)![]() in different pH
values. [Fe(phen)
in different pH
values. [Fe(phen)![]() ]
= 5 × 10-3 mol L-1, temperature = 45℃, [EP[ = [NE] = 1 × 10-5 mol L-1(in the
cell), upper panel—EP, lower panel—NE.
]
= 5 × 10-3 mol L-1, temperature = 45℃, [EP[ = [NE] = 1 × 10-5 mol L-1(in the
cell), upper panel—EP, lower panel—NE.
The overall reactions can be represented as follows:
A + mO![]() B + mR
(1)
B + mR
(1)
B + nO![]() C + nR
(2)
C + nR
(2)
Where A represent EP or NE, B represent the intermediate product of EP
or NE, and C represent the final product of EP or NE. O is Fe(phen)![]() which react with A or B to give R. m and n
counts for the stoichiometry of the two reactions respectively, also refers to the
electrons lost by A and B, m+n refers to the total electrons lost when A converted to C. k1
and k2 are pseudo-first order rate constants when O remain far excess amount
over mA+nB through out the reaction. The kinetic equation of B and C is well known as
follows[6]:
which react with A or B to give R. m and n
counts for the stoichiometry of the two reactions respectively, also refers to the
electrons lost by A and B, m+n refers to the total electrons lost when A converted to C. k1
and k2 are pseudo-first order rate constants when O remain far excess amount
over mA+nB through out the reaction. The kinetic equation of B and C is well known as
follows[6]:
[B]t = [A]0![]() (e
(e![]() - e
- e![]() )
)
[C]t = [A]0 (1-![]() (k2e
(k2e![]() - k1e
- k1e![]() ))
))
from the fact that
[R]t = m[B]t + (m + n) [C]t
we deduced the kinetic equations of R as follows:
[R]t = [A]0 ((m + n) +![]() e
e![]() +
+![]() e
e![]() )
)
where [B]t, [C]t, [R]t are the concentration of B, C, R,
at time t, [A]0 is the initial concentration of A.
In order to confirm the above mechanism, the experimental kinetic data
obtained at different conditions are fitted to the kinetic equation of R, m and n are
obtained by trying a series of integer numbers, and the best value is accepted at which
other parameters are calculated in the smallest least squares. Some of the typical kinetic
curves are given in a previous study[7], some of the unreported are given here
in Fig.2 It can be seen that kinetic data obtained at different conditions are all
agreeing well with the theoretic kinetic equation of R. The parameters are summarized in
Table1.
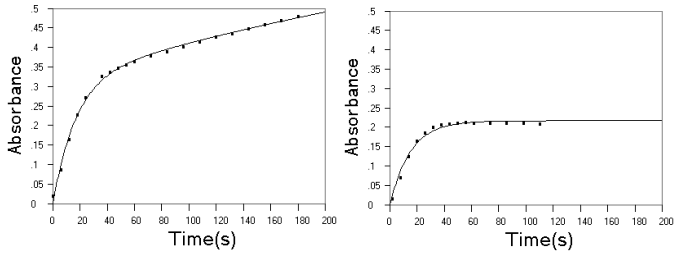
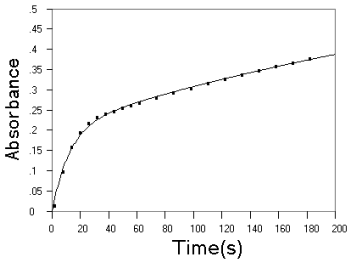
Fig.2 Experimental (■) and fitted (--) kinetic curves on reaction of EP and NE with
Fe(phen)![]() . Upper
panel, EP at pH 1.2; middle panel, NE at pH 1.2; lower panel, NE at pH 3.86. Other
conditions as described in experimental section.
. Upper
panel, EP at pH 1.2; middle panel, NE at pH 1.2; lower panel, NE at pH 3.86. Other
conditions as described in experimental section.
Table 1 Parameters for
reactions of EP and NE with Fe(phen)![]()
Conditions |
k1/s-1 |
k2/s-1 |
m |
n |
m + n |
pH 1.2 ,NE |
6.698 × 10-2 |
0 |
2 |
0 |
2 |
pH 1.2, EP |
7.955 × 10-2 |
2.449 × 10-3 |
2 |
4 |
6 |
pH 2,NE |
1.990 × 10-1 |
2.744 × 10-3 |
3 |
1 |
4 |
pH 2, EP |
1.554 × 10-1 |
1.797 × 10-2 |
5 |
3 |
8 |
pH 3.86 ,NE |
4.949 × 10-2 |
1.386 × 10-3 |
3 |
5 |
8 |
*pH 4.25 ,NE |
7.986 × 10-2 |
1.185 × 10-2 |
3 |
4 |
7 |
*pH 4.25, EP |
9.799 × 10-2 |
2.069 × 10-3 |
6 |
2 |
8 |
pH 4.6 ,NE |
2.854 × 10-2 |
2.865 × 10-2 |
5 |
1 |
6 |
pH 4.6, EP |
9.493 × 10-2 |
1.924 × 10-3 |
5 |
1 |
6 |
* [Fe(phen)![]() ] = 1.00 × 10-2 mol L-1
] = 1.00 × 10-2 mol L-1
The m and n values in Table 1 offers important information
about the intermediate products formed in the oxidation reaction of EP and NE. According
to the electrons lost, an oxidation pathway is proposed as in Scheme 1.
This mechanism is different from literature[8] in three
aspects: (1) proposed intermediate products that lost
3, 5, 7 electrons given as 3, 5, 7, (2) produce products that lost 6 electrons (6) from products
that lost 4 electrons (4), but not via 3,5,6-trihydroxyindole species, (3) The final product that lost 8 electrons (8).
Although a common pathway is proposed, EP and NE behave differently due
to the difference in structure. At pH 1.2, EP and NE lost 2 electrons first(2),
then EP go on oxidation to form a product that lost 6 electrons(6), but the
oxidation of NE is stopped. This is probably because in structure of NE, a hydrogen atom
is substituted for methyl to connect with nitrogen atom, thus at acidity of pH 1.2, the
protonation of nitrogen is so strong that the oxidation can not go on, while in the case
of EP, because the methyl group connected to nitrogen have the effect of electron
repelling and position hindering, the protonation of nitrogen is thus weaker and electron
is easy to be lost, so the oxidation of EP at low pH value can go on.
At pH 2, EP first form an intermediate product that lost 5 electrons(5),
then go on oxidation to form a product that lost 8 electrons(8). NE pass the
product that lost 3 electrons(3) and form an oxidation product that lost 4
electrons(4). At pH 3.86, the oxidation of NE pass the product that lost 4
electrons to give a final product that lost 8 electrons(8).
At pH 4.25 the concentration of Fe(phen)33+ is
doubled, EP first form a product with 6 electrons lost(6), then give a final
product with 8 electrons lost. NE first form product that still lost 3 electrons(3),
and the final product that only lost 7 electrons(7).
At pH 4.6, the electrons lost by final products of both EP and NE are
only 6, and the electrons lost by the first stage products of both EP and NE are 5.
The structure of 2 to 8 in oxidation pathway were
proposed by us only to fit the electrons they lost, other mesomeric structure may be
favorable, for example, the structure of 2', 4',
6' and 7' has been proposed by others [9,10], and may be the
mesomeric structure for 2, 4, 6 and 7 respectively.
The need of acid to form the product of 6' may
be used to explain our foundlings that at lower pH, final products with 8 electrons lost
could be formed, but at higher pH, no such final product was formed. The detail mechanism
for this observation is still unknown.
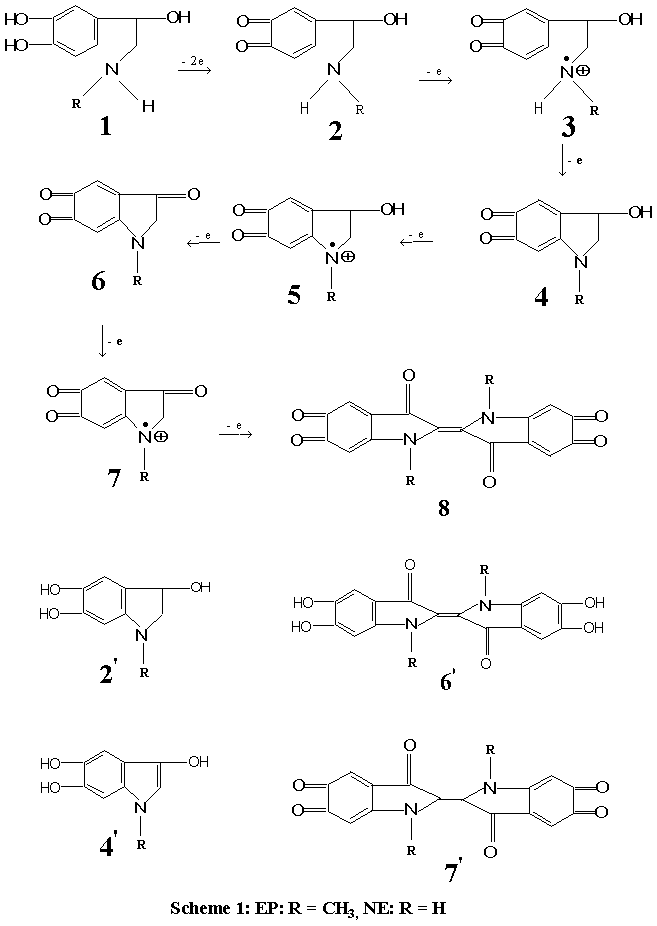
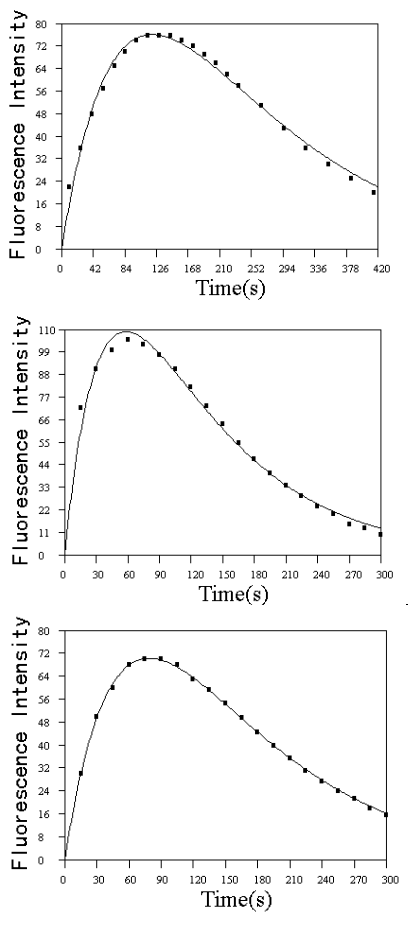
Fig.3 Experimental (■) and fitted (--) kinetic curves on reaction of EP with dissolved O2
in alkaline solution. Upper panel, 30ºC
0.5 mol L-1 NaOH; middle panel, 40ºC 1 mol L-1
NaOH; lower panel, 40ºC 0.1 mol L-1 NaOH. Other conditions as described in
experimental section.
2.2 Reactions with O2 in alkaline solution
EP and NE were found to react with dissolved oxygen in alkaline solution to give a
highly fluorescent species in the presence of a proper reducer, such as ascorbic acid
etc.. The maximum wavelength of excitation (lex) was found to be 410 nm, and that of emission (lem) was 510 nm. The
fluorescent species was known as trihydroxyindole species and the structure was also known
(4'). When no reductant was present, the trihydroxyindole species was unstable and
went on further oxidation to products with no fluorescence, so the fluorescence intensity
of the solution decreased. The structure of the final product is unknown, but may be as
oxoaminochrome (6). The overall process can be followed by monitoring the variation
of fluorescence intensity with time using FISF technique. A number of kinetic curves were
obtained for EP by this method at different conditions and the kinetic data were fitted to
the kinetic equation of B, good results were obtained and reported in a previous paper[11].Some
of the unreported results are given in Fig.3. The rise and fall of the fluorescence
intensity confirms the successive reaction of O2 with EP, which can be used in
kinetic analysis and to study oxidation pathway.
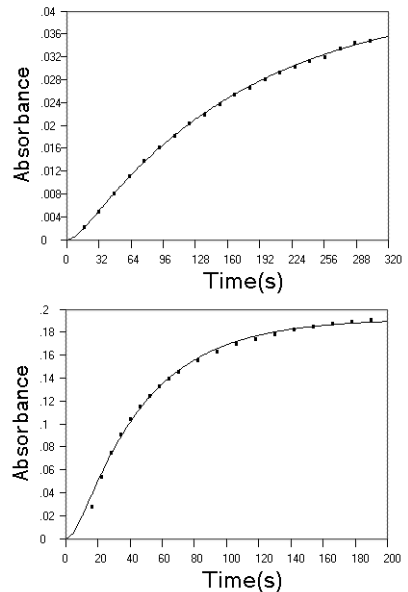
Fig.4 Experimental data (■) and fitted kinetic curve (--) on the reaction of EP (upper panel)
and NE (lower panel) with Fe(CN)63-. Experimental conditions for EP:
[EP] = 2 × 10-5 mol L-1, [Fe(CN)63-] = 4 ×
10-3 mol L-1, pH = 3; for NE: [NE] = 1.563 × 10-4 mol L-1,
[Fe(CN)63-] = 8 × 10-2 mol L-1, pH = 4.5.
Temperature = 40ºC.
2.3 Reactions with Fe(CN)63-
in weakly acid media
EP and NE were found to react with Fe(CN)63- in weakly acid
media to form red products which exhibit an absorption peak at 490 nm. The products have
long been known as aminochromees(4). The reactions can be followed by monitoring
the variation of absorbance with time using FISF technique. A number of kinetic curves
were recorded for EP and NE at different pHs and found that at lower pHs, an obvious
induction period was observed at the start phase of the oxidation reaction; but at higher
pHs, the induction period was diminished and the kinetic curve can be explained by simple
first order kinetics. After the reaction has been reached equilibrium, no absorption
decrease at 490 nm was observed, indicating that the reaction did not pass the aminochrome stage. This is different from the fact that
in alkaline solution, without the protection of a reductant, the reaction of EP and NE
with Fe(CN)63- pass the fluorescent trihydroxyindole stage, which is
the rearrangement form of aminochrome, to form a further oxidation product with no
fluorescence [6]. The kinetic curves with an obvious induction period can be
explained by the successive reaction kinetics with detection of final products. Fig.4 was
the experimental kinetic data and fitted kinetic curve using kinetic equation of C. As can
be seen, the kinetic data was fitted well, the rate constants for EP at pH 3 were: k1
= 0.111 s-1, k2 = 0.0254 s-1 and for NE at pH 4 are: k1
= 2.697 × 10-2 s-1, k2 = 9.726 × 10-3 s-1.
This was exploited in a two-rate method for the simultaneous determination of EP and NE [12].
The presence of successive reactions indicated that an intermediate product was formed
during the course of developing the final product of aminochrome. According to the
oxidation pathway, the intermediate must be 2. This is supported by the observation
that a sharp increase in initial rate is present for EP at pH about 4, and for NE at pH
about 5 (Fig.5). The fact that higher pH is needed for the rapid oxidation of 2 in
the case of NE agree with the structure difference between EP and NE, as explained in
previous section.
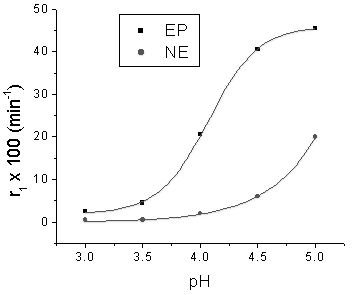
Fig.5 Effect of pH on the initial rate of EP and NE.[NE] = [EP] = 2 × 10-5
mol L-1, [Fe(CN)63-] = 8 × 10-2 mol L-1,
Temperature = 40ºC.
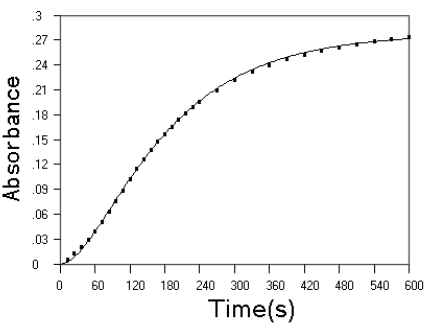
Fig.6 Experimntal kinetic data (■) and fitted kinetic curve (--) on the reaction of NE with HRP/H2O2
system. Conditions as desribed in experimental section.
The HRP catalyzed reaction of H2O2 with EP and NE was studied in the phosphate buffer of pH 7.4. red products were formed with one absorption peak at 490 nm and charaterized as aminochrome (4). The reaction was followed by the FISF technique and found that an induction period was observed for NE, but not for EP. When the reaction has reached equilibrium, no dcrease of absorption at 490 nm was observed for both EP and NE within 10 min.. This indicated that the reaction did not pass the aminochrome stage. The kinetic data of NE can be fitted to kinetic equation of C very well (Fig.6), indicating that an intermediate product is present during the course of devoloping the red products. Althrough no further efforts was done to elucidate the intermediate, it must probably be 2 in oxidation pathway proposed in previous section. The rate constants for NE is: k1 = 1.660 × 10-2 s-1, k2 = 7.059 × 10-3 s-1.
3 CONCLUSION
The successive reactions of EP and NE with different oxidizers were confirmed
in this paper by fitting the kinetic data to the coresponding successive kinetic
equations. The presence of intermediate product was naturally indicated by the presence of
a successive reaction. Additional information about the oxidation state of the
intermediate and final products could be obtained by studies on the kinetic of detecting
the reagent in successive reactions, as described in the case studies of the reaction of
EP and NE with Fe(phen)![]() . This make us
realized that the complex oxidation pathway could be investigated by using the kinetics of
successive reactions. It must be emphasized that the oxidation pathway and structures
proposed for EP and NE were merely deduced based on results from kinetic studies, it may
be the correct one, but the validity must be confirmed by further studies.
. This make us
realized that the complex oxidation pathway could be investigated by using the kinetics of
successive reactions. It must be emphasized that the oxidation pathway and structures
proposed for EP and NE were merely deduced based on results from kinetic studies, it may
be the correct one, but the validity must be confirmed by further studies.
[1] Bu'Lock J D. J. Chem. Soc., 1961, 52.
[2] Zhang YB, Dai JB, Jiang DQ, et al. Chem. J. Chin. Univ. (Gaodeng Xuexiao Huaxue Xuebao), 1992,13 (2): 195.
[3] Dai JB etc. Chem. J. Chin. Univ. ( Wuli Huaxue Huaxue Xuebao), 1991,12 (3): 260.
[4] Kalyanaraman B, Felix C C, Sealy R C. J. Biol. Chem., 1984, 259: 354.
[5] Mieling G E, Pardue H L. Anal. Chem., 1978, 50: 1611.
[6] Benson S W. The Foundations of Chemical Kinetics, New York: McGraw-Hill, 1960.
[7] Wu X G, Cai R X. Analyst, 2001, 126: 690.
[8] Vochten R F C, Hoste J, Delavnois A L, et al. Anal. Chim. Acta, 1968, 40: 447.
[9] Heacock R A. Chem. Rev., 1959,59: 181.
[10] Harley-Mason J J. Chem. Soc., 1950,1276.
[11] Cai RX,Wu X G, Liu Z H, et al. Analyst, 1999, 124: 751.
[12] Wu XG, Cai RX, Zhang L, et al. Anal. Chim. Acta, 2001,448: 257.
利用连串反应动力学研究肾上腺素和去甲肾上腺素的氧化过程
吴新国1,蔡汝秀2,林智信2
(1武汉大学资源与环境科学学院,湖北武汉,430079; 2武汉大学化学与分子科学学院,湖北武汉,430072)
摘要 对肾上腺素和去甲肾上腺素与各种氧化剂的反应动力学进行了大量研究,发现这两种儿茶酚胺与各种氧化剂的反应都具有连串反应的特征。在不同氧化剂、不同pH或其它不同条件下,生成的中间产物和终产物也都不同。根据以上结果提出了反应历程。两种儿茶酚胺的氧化动力学可以用连串反应动力学模型很好地解释。
关键词 氧化历程,连串反应,肾上腺素,去甲肾上腺素。