http://www.chemistrymag.org/cji/2005/079062pe.htm |
Sep. 22, 2005 Vol.7 No.9 P.62 Copyright |
Sorption and desorption of 2,4-dichlorophenol in soil environment
Zhu Kun 1, Jiang Mei2,
Zhan Huiying3
(1Department of Environmental Eng., Lanzhou Jiaotong University, Lanzhou,
730070, China; 2Guangzhou Chemical Technology College, Guangzhou, Guangdong
510370, China; 3Department of Chemistry, Gansu Lianhe University, Lanzhou,
730000, China )
Received on Jun.29, 2005; Supported by the National Natural Science Foundation of China (No.29977015) and Science Foundation of Gansu Province (No.GH2002-14).
Abstract The
sorption-desorption processes and mechanisms of 2,4-dichlorophenol (DCP) on the soil
surfaces were studied by using column tests. The results proved that the isothermal
sorption curves of DCP were nonlinear, and could be explained with Freundlich Equation.
The sorption rates of DCP in the soils increased with the increase of the initial
concentrations, and were positively related to the calculated Freundlich constant (KF)
and the adsorption capacity of soil (Csmax). Moreover, the desorption behaviors
of DCP in the soil environment showed the significant retention that was affected by pH
and the existence of the metal cations in the environment. Sorption of DCP could be
increased when pH decreased from 10.8 to 6.7, indicating that the sorption of neutral DCP
was higher than its ionic form in the soil matrix as DCP is an ionizable compound (pKa=7.7).
At the high pH values, most of the neutral molecules could be ionized to be free ions or
ion pairs that have the stronger hydrophilicity and easier enter aqueous phase. The
apparent sorption retention of DCP on the soils could be observed if the soils contain
two-valence metal cations. It was found that DCP retention was enhanced by cations
following the sequence as: Cd2+ > Mn2+ > Zn2+ >
Ca2+ > Mg2+. Sorption and desorption of DCP in the soil matrix
can be described by an extended Dual-Mode Model.
Keywords 2,4-dichlorophenol; Sorption, Loess soil, Retention,Column test
1. INTRODUCTION
Chlorophenols are a group of chemicals in which chlorines (between one and five) have
been added to phenol. There are five basic types of chlorophenols in which
2,4-dichlorophenol (2,4-DCP) as a di[two]chlorophenol, has a wide environmental concern
because it is commonly used as mothproofing and miticide. The largest uses for 2,4-DCP
have also been found as an intermediate, especially in the productions of herbicides and
pesticides. Man who is exposed to 2,4-DCP, could through breathing cause risk of cancers,
and through the skin developed acne cause mild injure to his livers [1].
2,4-DCP can be present in drinking water when chlorine is used to disinfect it, therefore,
the most likely source from which children could be exposed to 2,4-DCP, is water that has
been disinfected by chlorine.
Children may also be harmed in areas where has been sprayed as
herbicides and pesticides. Volatilization may be the major dispersal mechanism of DCP into
the atmosphere, as a result, it has often been detected in atmospheric emissions from the
combustion of municipal solid waste, hazardous waste, coal, wood, and 2,4-DCP-based
herbicides[2]. It was reported that 2,4-DCP was also found in the leachate from
an industrial landfill [3]. Moreover, some chlorophenols, such as 2,4,5-T that
is often used as herbicide on food crops, can break down to form 2,4-DCP. Therefore it has
often been detected both in surface water and groundwater in addition to various aqueous
systems and terrestrial systems.
However, the majority (85%) of known environmental releases of 2,4-DCP
were to surface water through effluents discharged from industries that manufacture iron
and steel, electrical components, photographic processing, pharmaceuticals, organic
chemicals and from paper & pulp mills[4,5]. Due to the great solubility and
high mobility and wide distribution in the environment, 2,4-DCP has been taken as one of
the priority pollutants in effluent by USEPA[6] that regulates a wastewater
discharge limitation 112mg/L for one day,and monthly
average maximum concentration should not exceed 39mg/L under the best available treatment
technology while 0.3 mg/L for the drinking water quality standard and 3.08mg/L for both
ambient water quality criteria and fishery water. The environmental fate and transport of
2,4-DCP are controlled by its physical and chemical properties and environmental
conditions. Volatilization of 2,4-DCP from water is expected to be slow and, therefore,
not a major removal process from surface waters. The biological treatment of waste
containing 2,4-DCP has showed that none of the chemicals is removed by stripping[7].
Volatilization from topsoil is also not expected to be a significant removal process.
Thus, the environmental fate and transport of 2,4-DCP has the tendency to partition into
sediments and lipids and to bioconcentrate.
As an anthropogenic organic compound, 2,4-DCP is usually regarded to be
a polar or hydrophilic chemical due to the H-bonding ability of the hydroxyl group
although it has the significant hydrophobic surface areas. Moreover, the hydroxyl group
permits orientational interactions with the sorbent. According to the chemical properties,
DCP can be prevented from the environmental contamination in transport in the soil-water
matrix, particularly regarding its sorption capacity that is the most important mechanism
for the attenuation of organic pollutants in the natural loess soil.
Sorption onto the soil surfaces and aquifer media is the key process
that controls mobility, transformation and biological degradation of organic contaminants
in the water-soil environment. Publications have reported on sorption of HOCs to soil and
sediments in aqueous systems, indicating that sorption isotherms follow both linear and
nonlinear equations[8]. Linear isotherms are primarily considered to be
resulted from partitioning (dissolution) of HOCs into the matrix of soil-organic matter.
On the other hand, the sorption isotherms could become non-linear because of non-ideal
behavior, such as competition among solutes for sorption sites and sorption-desorption
hysteresis[9]. There are two schools of thoughts dealing with the cause of
isotherm nonlinearity: (1) retention by heterogeneous soil organic matter that contains
both "rubbery" (or soft) and
"glassy" (or hard) polymer-like sorption
domains[10], and (2) the presence of small quantities of high-surface-area
carbonaceous materials such as soot[11]. Sorption capacity and/or nonlinearity
have been shown to be a function of certain polarity indices of organic matter, i.e.
elemental ratios (O/C) and aromatic or aliphatic characters. Interpreting the shape of
isotherms is a commonly used macroscopic approach to assess the role of soil organic
matter in the sorption of HOCs. Xing and colleagues investigated the sorption of 2,4-DCP
on two soils by using a batch equilibration method, and concluded that the sorption
conformed the Freundlich isotherm, but the relevant constant (KF) and
the exponent (n) were altered in the different soils [9]. It was found that the
sorption of 2,4-DCP could not completely be resulted from partitioning (dissolution) of
solute into the matrix of soil-organic matter, and might be in association with
hole-filling process of the sorbate at specific sites within SOMs[12]. Sorption
behavior is further complicated due to the physical complexity and heterogeneity of soil
materials, but the mobility capability can, in general, be characterized by the
retardation factor (R) that is usually determined by the column tests and breackthrough
curves.
The objectives of this study were to examine the sorption-desorption
characteristics of 2,4-DCP and the transport behaviors in the natural loess soil with a
set of open flow-through columns that could closely simulate the corresponding field
conditions in an attempt to define its sorption mechanisms. Furthermore, it allowed to
access the reversibility of reaction mechanisms without the artificial disturbance in soil
phase. The results are useful to ascertain the transport and trasformation of 2,4-DCP and
other chlorophenols in loess soil, and can also be utilized in the remediation techniques
of chlorophenols-contaminated soils.
2. MATERIALS AND METHODS
2.1 Materials
A loess soil sample used in the experiments, was taken and sampled from the land
subsurface 50-60cm deep in an uncontaminated area in Lanzhou City, China.
The soil sample was textured, and the overall characteristics are: sand
18.75%, silt 53.85% and clay 27.4%; pH 8.1; organic carbon 0.101%, CEC 3.21cmol/Kg. The
organic carbon content (OC) was measured using K2Cr2O7-H2SO4
oxidation at 180-185oC in an oil bath according to the methodology reported by
Li et al[13]. The Cation Exchange Capacity (CEC) of the natural loess soil was
measured by atomic absorption spectrometry with Ca2+ as an index cation and 0.1
M BaCl2 solution as the extracting reagent.
Chemicals 2,4-dichlorophenol was used as the sorbate. The
compound is usually regarded as a polar hydrophobic organic compound (HOC), and its
solubility is defined as 4000mg/L with logKow 2.75 and pKa7.7. CaCl2 0.01mol/L was added as a mineral constituent to
adjust ion strength of the solutions while hydrochloric acid and sodium hydroxide were
used to adjust the pH values of solutions. A number of inorganic salts, such as magnesium
chloride, calcium chloride, zinc chloride, manganese chloride and cadmium chloride were
respectively used to investigate the influence of divalent metal cations on the retention
of 2,4-DCP in the loess soil. All the chemicals used in this study were analytical grade,
and the solutions were prepared by using distilled water.
2.2 Sorption procedures
Batch tests were conducted at the pH range from 6.7 to 10.8. A weight of 5 g of
the loess soil was mixed with 50 ml of the respective organic compound solutions with
concentrations ranging from 2 to 200 mg/L in 50-ml Erlenmeyer flasks with glass caps. To
obtain sorption equilibrium, the soil-solution mixtures were initially stirred with a
vortex agitator and then placed on a shaker for continuous mixing at 200 rpm for about 24
h at 20oC in the dark. After equilibration, the samples were centrifuged at 3200×g for 20 min to separate aqueous and solid phases.
The DCP concentrations in aqueous phases were determined by a 7500 UV spectrophotometer
(Shanghai, China). Sorbed amounts of DCP were calculated as the difference between the
initial and final concentrations in aqueous phase. Blank sample without sorbate was also
prepared to determine the potential losses of solutes to flask walls and from processes
other than sorption.
2.3 Column tests
Column apparatus A set of thick-walled plexiglass columns 2.5cm
inside diameter and 50cm in length were prepared. Supported by a rack, a sieve-plate on
which a piece of Teflon filter screen was placed, was set at the bottom of each column.
The installation of column is illustrated in Figure 1. The air-dried loess soil of some
100g was slowly packed into each column and continuously compacted with a wooden rod until
the soil height approximately reached 12.5 cm. The bulk density was calculated as 1.63
close to that under the natural condition. The solution was distributed onto the top of
column with a syringe pump, and a relatively steady flow rate through each column was
desired under a stable hydraulic load and pressure.
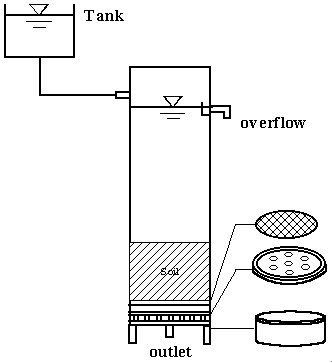
Fig 1. Illustration of installation for the column tests
Column tests The
soil packed in columns was initially wetted by the slow converse flow from the bottom with
a solution of 0.01M CaCl2 until the system was completely saturated, meanwhile
pH was adjusted until the effluents attained a constant pH value desired. Then, DCP
solutions with the different concentrations were respectively introduced into the three
columns with a steady flow rate of 10 mL·h-1. The effluents were
continuously collected from the outlet at each column bottom for the determination of DCP
concentrations and pH values. The column was then prepared
for the experimental displacement by passing a minimum of 50 pore volumes of the
experimental mobile phase through the column profile. Breakthrough curves were then
conducted by introducing a pulse of the solute containing mobile phase into the column and
monitoring column effluent until the solute effluent concentration C (mmol-1)
approached the influent concentration C0 (mmol-1), i.e. C/C0 =
1. Column effluent fractions collected from the H2O BTCs, was used to
characterize column hydrodynamics. Each column test was run in triplicate. The experiments
were conducted at the room temperature (20±1oC).
2.4 Analytical Methods
The effluent samples were centrifuged at 8000 rpm for 20 min to separate aqueous and
solid phase. DCP concentrations in the aqueous phase were determined by using a 754 UV
spectrophotometer (Shanghai, China) at the wave-length of 283nm where the interference of
background absorbance caused by eluted soluble humic materials could be omitted by the
comparison with the blank sample.
3. RESULTS AND DISCUSSIONS
3.1 Breakthrough curves of DCP on the soil column
Experiments were run with the three different concentrations of DCP solution 0.81,
0.55 and 0.33 mM at pH8.1 flowing respectively through the columns soaked initially by
CaCl2 solution of 0.01 mol/L. The effluent had the constant pH8.1 from the
beginning. Experimental data were calculated by the equation as:
Cw = f (v)
(1)
Where,Cw is the DCP
concentrations measured in effluent at different time, and v is the effluent volume.
The variety of DCP concentrations in the effluent was positively
related with that in the influent until the adsorption tended to be saturated. When
flushing with the fresh water, a significant retardation of desorption was observed due to
the curve shape with a long tail, which was one of the characteristics for non-linear
adsorption, and was reported by other batch experiments too[12].
As the columns of length 50 cm were packed with the 12.5 cm soil of
porosity 0.46, each column was pumped with water before the feed was switched to a
container containing DCP solution at the beginning. Assuming that chemical dispersion was
small, water front traveled much faster than the chemical due to its low adsorption to the
soil. The chemical front was sharp line as in plug flow, actually, DPC concentration
dropped off over a long distance because of the influence of dispersion and mass transfer.
If dispersion were negligible, the sorption would be linear and reversible in the study.
The definition of retardation factor could be expressed as the ratio of relative transport
velocity of water to contaminant in the soil matrix. When the sorption achieves
equilibrium under the dynamic state, R value can be calculated by the following equation:
![]() (2)
(2)
Where: u and uc are porous velocities
of water and contaminant, respectively; Ms - total mass of solid media; Vw
- Total volume of liquid in the soil metrix;![]()
![]() - Bulk density for porous medium; q - Effective soil porosity.
- Bulk density for porous medium; q - Effective soil porosity.
When steady state is reached, R can also be defined as [14]:
![]() (3)
(3)
Where, ms is the soil mass packed in the column, C0 is
the initial concentration of DCP in the injected solution, Vp is the total hole volume of
packed soil, and Ctot, ads is the total sorbed concentration in loess soil. It
can be seen that R is affected by the property of contaminant, so it is also related to
ratio of water velocity (u) to the contaminant velocity (uc).
On the basis of experimental data, the results were
shown as the breakthrough curves that are shown in Figure 2.
It can be seen that the sorption and desorption curves consist of two parts: the first
part is sharp and the second part is more diffuse and
spreading. It was also observed that the retardation of the sorption fronts depended on
the initial concentration, but the desorption curves was independent of the input
concentrations in a coordinate system when Cw reached 0.33mM and tended to be
decreased. At the same time, the observed adsorption-desorption breakthrough curves
appeared to be asymmetrical with the long-tails, which typically proved the DCP nonlinear
sorption and desorption hystereses[14]. According to the Extended Dual-Mode
Model, soil organic matter (SOM) has two states: the rubbery state and the glassy state.
The rubbery state looks like a partition sorbent and the sorption isotherm is linear,
however, it has many fixed or flexible holes and serves as the adsorption sites if the
polymer is glassy. Therefore, sorption of DCP on soil is a combination of portioning and
hole-filling adsorption. Sorption and desorption follow the same path when the polymer is
in the rubbery state. Inelastic expansion of holes or creation of new holes by penetrating
the enhanced expansion of soil surfaces at the glassy state may lead to stronger affinity
for adsorbate in the desorption process, and thus desorption hystereses occur.
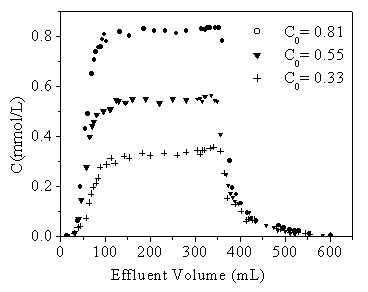
Fig 2. Breakthrough curves of DCP with three different
initial concentrations
(I=0.1M CaCl2 and pH=8.1).
3.2 Sorption isotherms
As the exact sorption equilibrium might be assumed at the time scale in the
experiments, the theory of sorption equilibrium could be used to interpret the results.
Freundlich and Langmur isotherms that are commonly used models to describe retention of
organic compounds onto heterogeneous sorbent, were developed to analyze the sorption data[15].
Here, Langmuir model has the form as:
![]() (4)
(4)
Where, Cs is the sorbed concentration of DCP (mmol/kg); Cw
(mmol/L) is the aqueous concentration; KL is Langmuir constant (mL/mol), and Csmax(mmol/kg)
is the adsorption capacity.
Finally, Freundlich model can be expressed as:
Cs= KFCw1/n
(5)
Where, KF is Freundlich constant that is an index to the sorption
capacity of sorbent, and 1/n is the parameter indicating the nonlinearity of isotherm.
On the basis of the experimental data, the best-fitting curves were
drawn and regressed by nonlinear analysis using the least-squares method [16].
The results obtained by both Freundlich and Langmuir equations are shown in Figure 3, and
the relevant parameters of the two equations are listed in Table 1. It could be seen that
the experimental data had a better fit for Freundlich Equation than for Langmuir Equation,
especially at the high initial concentrations. DCP sorption
was well described by the Freundlich-type isotherm, indicating the sorption equilibrium
under the concrete experimental conditions. As indicated by the values of KF
and Csmax, the sorption of DCP became stronger with the increase of initial
concentrations.
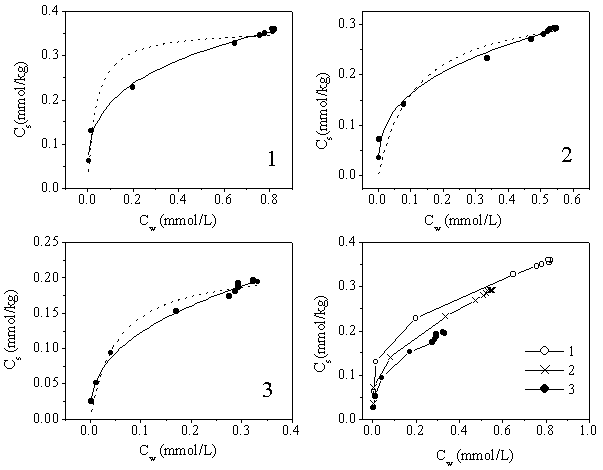
● experimental
data; —,Freundich fit line; ------,Langmuir fit
line.
Fig 3. The isotherms for 2,4-dichlorophenol (DCP) sorption on the loess soil with
the different initial concentrations (Curve 1 - 0.88mM; 2 - 0.55 mM; 3 - 0.33mM.)
Table 1 Freudlich-type and Langmuir-type parameters calculated with the different initial concentrations in the column tests
Initial |
Freundlich parameters |
Langmuir parameters |
||||
KF |
n |
r2 |
KL |
Csmax |
r2 |
|
0.82(mM) |
0.37829 |
0.29081 |
0.99238 |
29.22 |
0.36016 |
0.92404 |
0.55(mM) |
0.35394 |
0.33832 |
0.99273 |
8.732 |
0.34579 |
0.94249 |
0.33(mM) |
0.30064 |
0.38977 |
0.99485 |
19.17 |
0.21893 |
0.97827 |
The relationship
between the Freundlich exponent (n) and the solution concentration was investigated by Xia
and Pignatello in detail through a series of experiments with apolar
or polar HOCs including DCP [17]. In most cases, n presents the near
unity at a low concentration declining to a minimum value (nmin). Thus it was concluded that
nonlinearity, as defined by the Freundlich exponent n, was markedly sensitive to the
concentration range. The values of n for DCP adsorptive isotherms were defined from the
near unity to 0.5 under the different DCP concentrations. In our experiments, Freundlich
exponent (n) that decreased with the increase of initial concentrations, was matched with
the altering tendency regulation observed by Xia and colleague[17]. However,
the values of n measured as 0.29-0.38 in our experiments, were lower than that they did.
The reason could be attributed to the relatively lower level of organic carbon in the
natural loess soil.
3.3 Influence of pH
The effect of pH on the retention of DCP in the soil environment was studied at an
alternative pH range from 7.2 to 9.8 that is the typical pH range of the natural loess
soils in northwest China. Figure 4 shows the comparison of DCP breakthrough curves using
the different solutions at pH 7.2, 8.1 and 9.8 respectively under the identical
conditions. It was proved that the adsorption approached equilibrium more quickly at the
higher pH values. Thus, sorption of DCP tended to decrease with the increase of pH values.
For instance, the sorption equilibrium occurred at pH9.8 when the effluent volume of 100ml
was collected, but at the same situation it was found at pH 7.2 only when the effluent of
150ml was collected. Similarly, the retention appeared more significant with the increase
of pH values when the fresh water flushed the column soil. As DCP is an ionizable compound
(pKa=7.7), most of the neutral molecules could be ionized to be the free ions
or ion pairs that have the stronger hydrophilicity in the soil environment and easier
enter aqueous phase[18,19].
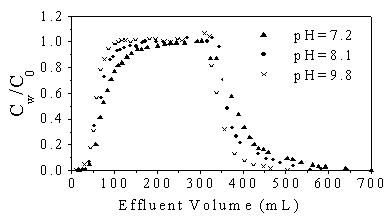
Fig .4 Effect of pH on breakthrough of DCP (DCP concentration 0.55 mM and I=0.1M CaCl2)
3.4 Influence of divalent metal cations
To affix the ion strength and species of cation in the experimental soil, CaCl2
solution of 0.01mol/L was used to saturate the column soil. Previous studies indicated
that metal cations on soil surfaces, especially intermediate( transition) metal cations,
could affect the sorption and desorption behavior of organic compounds in the soil matrix
or natural solids [18]. DCP of 0.55 mol/L was used as a sorbate in the
experiment while the soil was saturated with 0.1mol/L Mg2+, Mn2+, Zn2+
and Cd2+, respectively to replace Ca2+. Then the experiments of
sorption and desorption were conducted under the condition of pH8.1, and the results are
shown in Figure 5. It can be found that the appropriate sorption retention of DCP on the
soil was observed in the presence of Zn2+, Mg2+ and Ca2+
although alkaline metal cation Ca2+ and Mg2+ almost had the similar
effects on the behaviors of DCP. It was evident that DCP retention was enhanced by cations
following the sequence as: Cd2+ > Mn2+ > Zn2+ >
Ca2+ > Mg2+.The reason could be explained as follows: the
transition metal cations that occupied the adsorptive location, had higher chelating
capacity and were to form ligand bound with DCP, as a result, the desorption capacity
decreased.
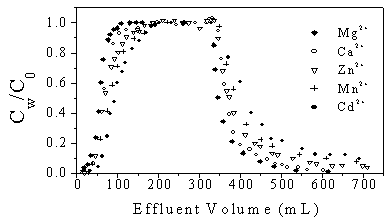
Fig. 5 Effect of metal cations on breakthrough curves
of DCP
(DCP concentration 0.55 mM, pH=8.1, metal concentration = 0.1M)
The total sorbed
concentration of DCP was calculated by
Cs = (Qtotal - Qout)/ms
(6)
Where, ms is the mass of loess soil sample, and Qout as
the amount of eluted DCP, was calculated by numerical integration of the areas under the
breakthrough curves when plotting Cw versus accumulated outflow V [20]:
Qout = ![]() (7)
(7)
The losses of compound by photochemical decomposition, volatilization
and the quantity adhered to column wall were neglected. Mass equilibriums in the sorption
and desorption reactions must be related with the influence of pH and metal cations in the
loess soil, and the results are shown in Tables 2 and 3, respectively. Both the sorbed and
released amount verified that DCP sorbed onto the loess soil surfaces was quantitatively
related to the eluting ion, and the sorption phenomena
involved were fully reversible.
On the basis of experimental data, retardation factor (R), which expresses the delay of
DCP mobility in the soil, was calculated by Equation 2.
Table 2 Mass balances and retardation factors affected by pH values in column tests
pH |
DCP sorbed (mmolkg-1) | Retardation factor ( R) |
|
Adsorption |
Desorption |
||
7.2 |
0.375 |
0.378 |
1.89 |
8.1 |
0.292 |
0.296 |
1.70 |
9.8 |
0.242 |
0.245 |
1.58 |
Table 3 Mass balances and retardation factors affected by metal cations in column tests
Background cation |
DCP sorbed (mmol/kg) |
Retardation factor(R) |
|
Adsorption |
Desorption |
||
Mg2+ |
0.250 |
0.252 |
1.59 |
Ca2+ |
0.292 |
0.296 |
1.70 |
Zn2+ |
0.350 |
0.357 |
1.83 |
Mn2+ |
0.4002 |
0.392 |
1.94 |
Cd2+ |
0.469 |
0.466 |
2.10 |
According to the theory on mass transfer through a natural material, DCP displayed a poor affinity onto the natural loess soil, so we could conclude that the presence of polar HOCs in the vadose zone, such as DCP, had a potential risk for groundwater contamination. However, the decrease of pH and the presence of small amount of metals could enhance the adsorption of DCP in the loess soil and prevent its mobility from topsoil into groundwater aquifer.
4. CONCLUSIONS
Through a series of the column tests in the laboratory, the natural loess soil was
used as a sorbent to study the sorption-desorption behaviors of DCP in the soil matrix.
The tests were prepared to be closer to the exact field conditions. The typical nonlinear
of sorption and desorption was hysteresed for DCP in the soil, and the results well fitted
the extended Dual-Mode Model when the sorption data of DCP better obeyed to Freundlich
isotherm relationship than Langmuir isotherm. The retention of DCP in the soil was
affected by pH and the presence of metal divalent cations. With a low retardation factor
in loess soil, DCP displayed a poor affinity on the loess soil surfaces. It is proposed
that the disposal of DCP into the vadose zone consisting of loess soil might have a
potential threat to groundwater quality. In general, these results provide the evidence
and information for an environmental impact assessment and the further remediation in
Northwest China and the Central Asia where vast and thick loess soils are expended in
topography.
REFERENCES
[1] ATSDR, Toxicological profile for chlorophenols, Agency for Toxic Substances and
Disease Registry, US; 1999, Document No. 205-93-0606.
[2] Oberg T, Warman K, Bergstrom J. Chemosphere, 1989, 19(1-6): 317-322.
[3] Brown K W, Donnelly K C. Journal of Wast Haz Mater, 1988, 5: 1-30.
[4] USEPA. 1981, 118. EPA-440-4-81-008, NTIS PB81-219685.
[5] Paasivirta Z, Heinola K, Humppi T et al. Chemosphere, 1985, 14: 469-491.
[6] USEPA. 1992, file: 40 CFR 401.15.
[7] Stover E L, Kincannon D F. Journal of Water Pollut Contr., 1983, 55: 97-109.
[8] Shaw L J, Beaton Y, Glover L A. Environ. Sci. Technol., 2000, 34(22): 4721-4726.
[9] Xing B S, Pignatello J J, Gigliotti B. Environ. Sci. Technol., 1996, 30(18):
2432-2440.
[10] Xing B S and Pignatello J J. Environ. Sci. Technol., 1997, 31(3): 792-799.
[11] Chou C T, Kile D E, Rutheford D et al. Environ. Sci. Techol., 2000, 34(7): 1254-1258.
[12] Xing B S, Pignatello J J. Environ. Sci. Technol., 1998, 32 (5): 614-619.
[13] Li Y. Conventional Analysis of Soil Chemistry (Turang Huaxue Changgui Fenxi),
Beijing: Scientific Press, 1983: 74.
[14] Bueno M, Astruc A, Astruc M. Environ. Sci. Technol., 1998, 32(24): 3919-3926.
[15] Ortega-Calvo J J, Fesch C, Harms H. Environ. Sci. Technol., 1999, 33(21): 3737-3742.
[16] Sannino F, Filazzola M T, Violante A. Environ. Sci. Technol., 1999, 33(23):
4221-4225.
[17] Xia G. S and Pignatello J J. Environ. Sci. Technol., 2001, 35(1): 84-94.
[18] Morillo E, Undabeytia T, Maqueda C. Environ. Sci. Technol., 1997, 31(12): 3588-3592.
[19] Daughney C J, Fein J B. Environ. Sci. Technol., 1998, 32(6): 749-752.
[20] Thomsen A B, Henrikser K, Gron C. Environ. Sci. Technol., 1999, 33(17): 2891-2898.
2,4-二氯酚在土壤环境中的吸附和解吸
朱琨1,姜梅2,展慧英3
(1 兰州交通大学环境与市政工程学院, 兰州 730070 中国; 2广州化工技术学院,广州
510370 中国; 3甘肃联合大学化学系,兰州 730000 中国)
2005年6月29日收稿;国家自然科学基金项目(29977015)及甘肃省环保局科研基金项目(GH2002-14)资助。
摘要 利用土柱试验对2,4-二氯酚在土壤表面的吸附和解吸过程和机理进行了研究。试验结果发现2,4-二氯酚在土壤表面的吸附等温线为非线性,可以用Freundlich等温式来描述。并且2,4-二氯酚在土壤中的吸附率随其初始浓度的增大而增强,与计算的佛德里希常数(KF)和土壤的吸附能力(Csmax)成正比。同时2,4-二氯酚在土壤环境中的解吸行为有明显的滞后现象,并受土壤pH值和共存的金属离子的影响。当pH从10.8降低到6.7时,
土壤对2,4-二氯酚的吸附会增大,由于2,4-二氯酚是可离子化的化合物(pKa=7.7),在土壤介质中中性分子形态的2,4-二氯酚的吸附大于它的离子形态。而在较高pH时,大部分2,4-二氯酚的中性分子可被离子化而成为亲水性的自由离子或离子对,极易进入水相。若土壤中共存二价金属离子时,2,4-二氯酚会表现出明显的解吸滞后性。阳离子对解吸滞后的影响作用可按如下顺序:Cd2+
> Mn2+ > Zn2+ > Ca2+ > Mg2 +排列。2,4-二氯酚在土壤中的吸附与解吸的规律可用扩展的双模式模型来描述。根据在天然介质中的传质理论,2,4-二氯酚对土壤表现出微弱的亲和力,因此象2,4-二氯酚这类极性碳氢化合物在包气带土壤中会构成对地下水的潜在污染,但是只要降低土壤的pH值和掺加极少量的金属离子就可增强土壤对2,4-二氯酚的吸附性,从而防止对地下水的污染。
关键词 2,4-二氯酚,吸附,黄土,滞后现象,土柱实验