http://www.chemistrymag.org/cji/2005/07a065pe.htm |
Oct. 2, 2005 Vol.7 No.10 P.65 Copyright |
Liu
Pengyan1, Wang Yingfeng2
(1College of Chemistry and Environmental Science, Hebei University, Baoding
071002; 2 Department of Chemistry, Capital Normal University, Beijing, 100037)
Received on Jun.10, 2005; Supported by the Natural Science Foundation of Hebei Province (B2005000105) and Hebei University Research Foundation.
Abstract The residue analysis of
clenbuterol in swine tissue, such as meat, liver, kidney, and lung was developed.
Extracting conditions, selection of solid-phase extraction column and derivatization
conditions were studied on. Perchloric acid extraction followed by two clean-up steps,
such as extraction with isopropanol-ethyl acetate and solid-phase extraction with strong
cation-exchange (SCX) resins. The analyte is determined by GC-SIMMS as the
mono-trimethylsilyl derivative prepared using N,O-Bis (trimethylsilyl) trifluoroacetamide
(BSTFA) +Trimethylchlorosilane (TMCS) ( 99+1,v/v). The limit of detection spiked at 1mg/kg in tissue samples was less than
0.4mg/kg under the
signal-to-noise ratio of 3:1, and the average recoveries (%) were more than 73.4 %. The
RSD was less than 10% (n = 3). Under the selecting conditions, the analyte was
clean-up with only one SCX resins column, so that the cost was decreased, the time was
saved, and the procedure was simple. Moreover, the recovery and sensitivity were satisfied
the determination requirements. The method could be used for routine ananlysis of
clenbutrol residue confirmatory in animal tissues.
Keywords swine tissue, clenbuterol, acid extraction, SCX column cleanup,
determination
1 INTRODUCTION
Clenbuterol (4-amino-3, 5-dichloro-a-tert-butylaminomethylbenzyl alcohol
hydrochloride) is a beta-adrenergic drug used as a bronchial dilating agent for the
treatment of pulmonary diseases in humans and animals. It is especially used in the case
of chronic illness [1]. In the 80's, it was happened to discover that it can
promote muscle growth and reduce body fat and increase muscle mass. After that it has been
used as growth promoters in animal feed [2] and it is also extensively misused
in farm animals where high doses give rise to a preferential muscle to fat ratio resulting
in financial gain for the farmer. Therefore, residue of clenbuterol in edible tissues is
happened sometimes. Consequently, it is essential to have a sensitive, rapid, low cost and
reliable technique to detect the presence of these residues. Different analytical methods,
such as GC-MS [3-9], HPLC-MS [10, 11], ELISA [12, 13],
HPLC [14-16], capillary electrophoresis[17], chemiluminescence
sensor[18], and so on, have been described. In these methods urine samples are
mostly used for determination clenbuterol, however, animal tissues are fewer used [5,
10, 11, 14]. Animal tissues pre-treatment is more complex than that of urine because
tissues are solid so a homogenisation step will be needed to allow access of the
extracting solvent to the sample. Besides, clenbuterol must be isolated from the tissue
proteins by hydrolysis--alkaline hydrolysis [19], acidic hydrolysis [10,
14], enzymatic hydrolysis [11, 20], for example. Because
animal matrix contains components too much to remove well, two kinds of cartridge (C8
and SCX [21,22], C18 and SCX[5] ) are often used for
clean-up of clenbuterol residue or Bond Elut Certify column, a kind of mixed-phase
cartridge, is often used for the procedures[6,23]. By using these solid-phase
columns it not only can bring high cost, but also make pretreating procedure complex. And most of recoveries are lower than 70%. In this
paper, we describe a simple, sensitive and lower cost method, as well as satisfied the
with requirement of maximum residue limits (1mg/kg) and recoveries >70%. Because this method involved a simple
clean-up by adjusting pH 11, following extraction by isopropanol-ethyl acetate, the
extract got a better clean-up with only one SCX column.
We have studied the influence of the acidic hydrolysis and enzymatic
hydrolysis on extracting efficiency, and compared one SCX column with C18
column and two columns of C18 combining with SCX. Finally we selected the
acidic hydrolysis for extraction method, one SCX column for clean-up. The cost is lower,
the time is more saved, and the procedure is simpler by using this method.
2. EXPERIMENTAL
2.1 Chemicals and reagents
Clenbuterol HCl [2-tert.- butylamino-1-(4-amino-3,5-dichlorophenyl) ethanol
hydrochloride] (purity ≥ 98.5%) was obtained from
the national institute for the control of pharmaceutical and biological products (Beijing,
China). N,O-Bis (trimethylsilyl) trifluoroacetamide (BSTFA), trimethylchlorosilane, (TMCS)
and heptafluorobutyric anhydride (HFBA) were obtained from SPUELCO (USA). Butylboronic
acid (BBA) was obtained from Tokyo Kasei Kogyo Co. Ltd., (Tokyo, Japan), N,O-Bis
(trimethylsilyl) trifluoroacetamide (BSTFA) containing 1% of trimethylchlorosilane (TMCS)
and b-Glucuronidase, From Helix
pomatia were supplied by Sigma–Aldrich (St
Louis, MO, USA).
Ethyl acetate, isopropanol, methanol, and toluene were HPLC purity
reagent from Merck (Darmstadt, Germany). Sodium chloride for analysis was pretreated for
4h at 650oC. Sodium hydroxide, sodium dihydrogen phosphate,ammonia and perchloric acid (super purity grade) were obtained from
Beijing Chemicals Co. (Beijing, China). The reagents were analytical grade unless
indicated otherwise.
Purified water was produced with a Milli Q system from Millipore
(Massachusetts, USA).For sample clean up, 500mg (3mL) C18 and SCX columns from
Spuelco (USA) were used.
2.2 Standard solution
The stock standard solution of clenbuterol was prepared at concentration of 1.0mg/mL
by dissolving 10.2mg of clenbuterol HCl in 10.00mL methanol and was stable at < -18oC
for six months.
Working solution were prepared by dilution of stock solution with
methanol to give concentrations 10.0 ng/mL, 50.0 ng/mL, 100.0ng/mL and stored under
refrigeration (0-4oC).Phosphate buffer, 0.1mol/L, pH 6.0, was prepared by
dissolution of 12.0g NaH2PO4 in 1000mL of deionised water and
adjusting the pH to 6.0 with 2mol/L NaOH solution.
Perchloric acid, 0.2mol/L, was prepared by dilution of 83mL perchloric
acid with deionised water to 1000mL.
4% ammonia solution/methanol, was prepared by dilution of 4mL ammonia
with methanol to 100mL.
Ammonium acetate buffer, pH 5.2, was
prepared by dissolution of 1.45g NH4Ac in 1000mL
of deionised water and adjusting the pH to 5.2 with acetic acid.
2.3. Instrumental analysis
An Ultraturrax homogenator (IKA, Staufen, Germany) was used for tissue homogenisation.
Nitrogen Evaporator with OA-SYS Heating System was obtained from Organomation Associates,
Inc. (N-EVAPTM 112, USA). SIGMA 3K30 Laboratory Centrifuges was from Germany.
The vacuum manifold used for solid phase extraction (VISIPREPTM DL) procedure
was obtained from SUPELCO (USA). HP 6890 gas chromatograph and HP 5973N mass selective
detector (MSD) was obtained from Agilent Technologies (USA). GC was equipped with
electronic pressure control unit and an autosampler (G 2613A). Instrument control, data
acquisition and data processing were carried out with HP ChemStation plus Rev. A. 09. 01.
The column used was an HP 5MS (30m length×0.25mm i.d.×0.25 mm film thickness) from
Hewlett–Packard (CA, USA).
Samples were injected in the splitless mode (0.75 min delay) using
helium as the carrier gas at the flow rate 1.0 ml/ min. The injector and transfer line
temperatures were set at 220oC and 280oC, respectively. Oven
temperature was programmed as follows: initial temperature 70oC for 1min,
increasing at a rate of 25oC /min up to 150oC, held for1min, and
then increasing at a rate of 6oC /min up to 280oC, maintained at 280oC
for 5min. The mass spectrometer conditions were as follows: electron impact ionization
voltage 70 eV for both SCAN and selected ion monitoring (SIM) mode, and with a solvent
delay of 8 min. The ion source and quadrupoles temperature were 230oC and 150oC,
respectively.
2.4 Sample preparation procedure
2.4.1 Extraction of clenbuterol from tissue
Acidic hydrolysis
A 5.00g of tissue sample that was cut into small pieces was put into a 50mL
polypropylene centrifuge tube with stopper, 20mL of 0.2mol/L perchloric acid was added,
after that homogenised for 3min. Next, the sample was extracted in an ultrasonic bath for
20min, followed by 30min under 80oC water bath. After being taken out and
cooled to room temperature, the sample was centrifugated under 4500g for 10min at 10oC.
10mL of the supernatant was purred into another centrifuge tube, and adjusted pH to 10-12
with 2mol/L sodium hydroxide. After about 5g of sodium chloride being added and mixed, the
supernatant was extracted for 1min by a vortex blender using 10mL of mixture of
isopropanol and ethyl acetate (4:6, v/v), centrifugated for 10min under 4500g. The
extraction step was repeated twice. The extracts were then evaporated to dryness under a
stream of nitrogen at 70oC. The residue was dissolved with 1.0mL of 0.1mol/L
phosphate buffer (pH 6.0) and filtered over 0.45-mm filter unless the residues were dissolved completely.
Enzymatic hydrolysis
A 5.00g of tissue sample was put into a 50mL polypropylene centrifuge tube with stopper,
20mL of 20mmol/L ammonium acetate buffer (pH 5.2) was added,
homogenised for 5min, after that 50mL b-glucuronidase
was added and blended. The enzymatic hydrolysis was performed at 37oC for 18 h.
When cooled to room temperature, the sample was centrifugated under 4500g for 10min at 10oC.
Next steps were carried out according to acidic hydrolysis procedures.
SCX columns were conditioned by washing consecutively with 6mL of methanol, 3mL of
deionized water, 3mL of sodium dihydrogen phosphate buffer (pH 6.0) and 3mL of deionized
water in turn. Next, the samples were loaded on the preconditioned columns. The columns
were washed with 4ml deionized water and 4ml methanol and were dried under vacuum to
remove as much residual solvent as possible. Then the samples were eluted with 6ml of
ammonia solution/methanol (4:96, v/v), the eluent was then evaporated to dryness under a
stream of nitrogen at 60oC.
2.4.3 Derivatisation
The residue was dried in a desiccator for at least 20min before derivatisation. Then 50mL 1% TMCS/BSTFA (1:99, v/v) was added,
vortex mixed and kept at 80oC for 60min. Next the reagent was evaporated, and
the derivative was dissolved in 0.5mL toluene, vortex mixed and analyzed by GC-MS. The
series standard solutions were derivatized simultaneously.
2.4.4 Standard curve
0.10, 0.20, 0.50, 1.00, 2.00mL of standard solution (10.0ng/mL) was taken, respectively,
and the solvent was evaporated under the stream of nitrogen at 60oC water bath,
and derivatized under the described conditions above. The concentration of 2.0, 4.0, 10.0,
20.0, 40.0 ng/mL derivatives were obtained and analysed by GC-MS. Calibration cure was
constructed from response versus concentrations. Good linearity was observed for
derivative, R= 0.7144C + 1.1798, r = 0.9975. The linear range was 2.0-50.0 ng/mL.
3. RESULTS AND DISCUSSION
3.1 The conditions of extraction
Because the analyses are often combine with protein in the living, when extracted the
samples require enzymatic hydrolysis [5, 11, 20]. Keskin considered that clenbuterol seemed to be excreted free in urine
rather than conjugated [2]. We did experiment of enzymatic hydrolysis and
acidic hydrolysis with positive swine lungs, found there was no significant difference
between two results (listed in the Table 1) (a=0.05, tcal=0.34<ttable=2.78). This result
is similar to the literature [24].
However, the interfering compounds are more using enzymatic
hydrolysis than using acidic hydrolysis. Moreover, pretreated time is longer using
enzymatic hydrolysis. So it is optimum that the method of extraction using acidic
hydrolysis.
Table 1 Comparing acid hydrolysis with enzymatic hydrolysis (mg/kg)
1 |
2 |
3 |
means |
s |
|
acid hydrolysis |
7.64 |
7.70 |
7.85 |
7.73 |
0.108 |
enzymatic hydrolysis |
8.15 |
7.31 |
7.99 |
7.82 |
0.446 |
3.2 Solid phase extraction
Solid-phase extraction has been widely in use to analyze clenbuterol in biological
matrices. Two columns combining with or mixed-phase cartridges were often used in order to
obtain optimum clean-up, like describing in the part of introduction.
We have tried to contribute a simpler and lower cost clean-up method. In this
experiment C18, SCX and C18+SCX columns were used for cleaned up the
sample, respectively. Three columns were compared with each other. It was found that one
SCX column was the same as C18+SCX and had good clean-up efficiency, but C18
was not satisfied. So we selected one SCX column to clean-up. Because acid extract was
adjusted pH and extracted with isopropanol/ethyl acetate beforehand, SPE clean-up became
simple.
3.3 The elution condition of SCX cartridge
The solution of phosphate buffer of clenbuterol was added onto the conditioned SCX column,
then was washed and eluted under the conditions of describing
in "2.4.2 solid-phase extraction procedure". Every one milliliter of the eluent
was collected separated, and dried, derivated and analyzed. The result showed in Table 2.
It confirmed that clenbuterol was eluted completely by 6 mL of 4% ammonia/methanol.
Table 2 The results of eluent
elutents |
the first millimeter |
the second millimeter |
the third millimeter |
the fourth millimeter |
the fifth millimeter |
the sixth millimeter |
percents of elution (%) |
ND |
29.8 |
51.0 |
15.5 |
1.7 |
ND |
3.4 Derivatizing reagents
3.4.1 Silylation
The standard solution was evaporated to dry under nitrogen, 50mL of 1% TMCS/BSTFA was added, vortex
mixed and kept at 80oC for 60min. The reagent was evaporated under nitrogen,
and the derivative redissolved in 0.5mL toluene, vortex mixed, and analyzed by GC-MS with
both SCAN and selected ion monitoring (SIM) mode.
3.4.2 Esterification
The standard solution was evaporated to dry under nitrogen, 50mL ethyl acetate was added and vortexed. Then 50mL BBA was added and vortex mixed, kept
at 60oC for 60min. The solvent was evaporated under nitrogen, and the
derivative redissolved in 0.5mL ethyl acetate, vortex mixed and analyzed by GC-MS with
both SCAN and selected ion monitoring (SIM) mode.
3.4.3 Acylation
The derivative reaction with 50mL HFBA was carried on room temperature for 30min. The other
procedures were the same as above esterification. The acylation efficiency at room
temperature is better than at 40oC, and derivatization reaction is better with
ethyl acetate than without ethyl acetate.
Three derivative reagents were used, one which was BSTFA, a kind of
silylation reagent, another was BBA, a kind of esterification, the third was HFBA, a kind
of acylation. The results of derivatisation were given in Table 3 and Fig.1.
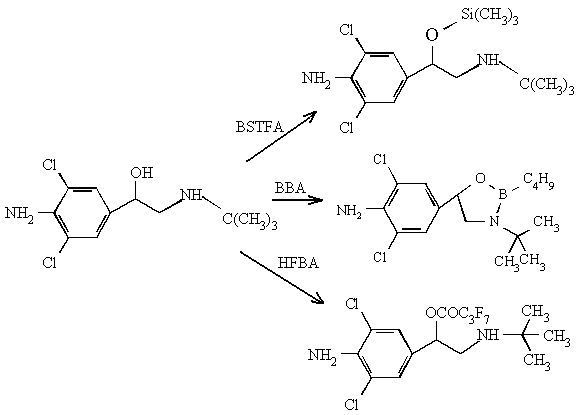
Fig. 1 Three derivatives of clenbuterol
The derivatives of clenbuterol-HFBA and clenbuterol-BBA (cyclic boronate
derivatives of clenbuterol) are formed the most abundant ions in the high mass region, and
there are strong characteristic. The sensitivity of clenbuterol-HFBA is lower than
clenbuterol-BBA and clenbuterol-BSTFA. Furthermore, HFBA is easy to form acid, thus result
in tailing factor. BBA is the same as HFBA. So, we have selected BSTFA as derivative
reagent. Its derivative has lower limits of detection, less impurities, and it is of good
volatile easy to remove.
Table 3 The data of three clenbuterol derivatives
DR |
MW |
RT(min) |
CI |
C(ng/mL) |
StN(S/N) |
LOD(ng/mL) |
BSTFA /TMCS |
348 |
15.56 |
86, 243, 262, 277 |
2 |
9.6 |
0.62 |
HFBA |
472 |
15.63 |
363, 398, 439, 456 |
25 |
4.8 |
15.6 |
BBA |
342 |
21.26 |
243, 245, 327, 329 |
5 |
24.3 |
0.62 |
DR: derivatisation reagents; MW:
molecular weight; RT: retention time; CI: Characteristic ions; C:
concentration; StN.: Signal-to-Noise; LOD: limits of detection
3.5 Comparation BSTFA with 1% TMCS/BSTFA
According to the report [25] the mixture of TMCS and BSTFA (1:99, v/v) has
stronger derivative effect than BSTFA alone. The result is good when making amino acid
derivate. The amount of formation of N,O-TMS was less than 1% when clenbuterol was derived
by BSTFA, but the amount of that is increased to 5% by 1% TMCS/BSTFA[26], and
therefore 1% TMCS/BSTFA was through of having high ability of amido sylilation. The study
was performed by comparing BSTFA with 1% TMCS/BSTFA though two concentration test, SCAN
and SIM two modes determination, and found there was not different for formation of O-TMS.
3.6 Recovery, precision and limit of detection
The precision and recovery of the method were determined by analyzing 5.00g samples of
swine tissue, which included meat, liver, lung, and kidney. Every kind of tissue was
spiked with clenbuterol at three different concentrations of 1.0, 5.0, and 20.0 mg/ kg respectively, and the sample
three repetitions were carried out for each fortification level. The sample was extracted,
cleaned up and analyzed under the above conditions. Profiles are shown in Fig.2. The
recoveries of the analytes such as meat, liver, lung, and kidney ranged from 70.4 % to
92.3, 73.4% to 98.8%, 79.4% to 107.0%, and 76.4% to 94.0%, respectively, and the relative
standard deviations (RSDs) varied from 2.3% to 7.5%, 6.7% to 7.5%, 0.9% to 9.4%, 2.9% to
7.2%. As shown in Table 4, the recoveries are all above 70% for four kinds of tissue.
Table 4 Recoveries(%) and RSDs (%) in different tissues
Spiked of levels |
Spiked meat ( mg/kg) |
Spiked liver ( mg/kg) |
||||
1.0 |
5.0 |
20.0 |
1.0 |
5.0 |
20.0 |
|
1 |
70.4 |
83.4 |
85.6 |
85.0 |
90.3 |
74.1 |
2 |
80.1 |
84.8 |
92.3 |
98.8 |
91.3 |
78.0 |
3 |
70.7 |
81.0 |
84.2 |
93.4 |
80.7 |
68.2 |
Means of recovery |
73.7 |
83.1 |
87.4 |
92.4 |
87.4 |
73.4 |
RSDs |
7.5 |
2.3 |
5.0 |
7.5 |
6.7 |
6.7 |
Spiked of levels |
Spiked kidney ( mg/kg) |
Spike lung ( mg/kg) |
||||
1.0 |
5.0 |
20.0 |
1.0 |
5.0 |
20.0 |
|
1 |
76.4 |
93.9 |
81.3 |
85.8 |
85.6 |
87.2 |
2 |
83.4 |
89.3 |
93.9 |
88.8 |
99.5 |
107. 0 |
3 |
85.6 |
94.0 |
86.5 |
79.4 |
84.3 |
86.0 |
Means of recovery |
81.8 |
92.4 |
87.2 |
84.7 |
89.8 |
93.4 |
RSDs |
5.9 |
2.9 |
7.2 |
5.7 |
9.4 |
0.9 |
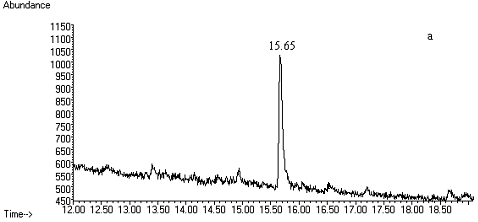
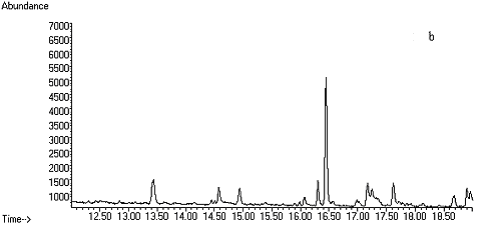
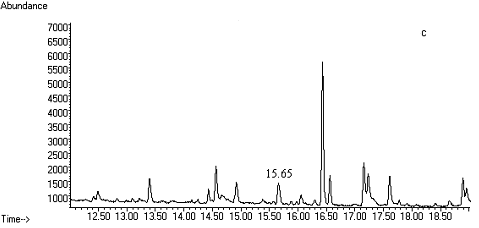
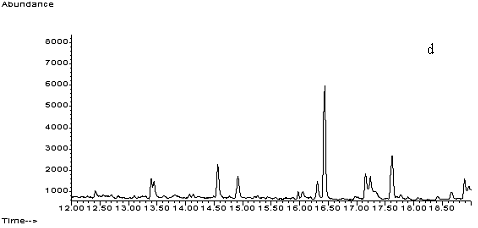
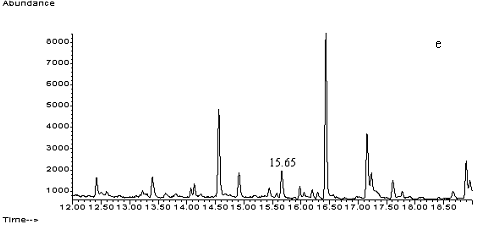
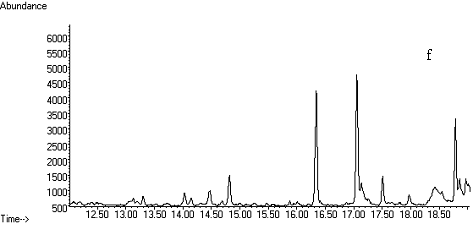
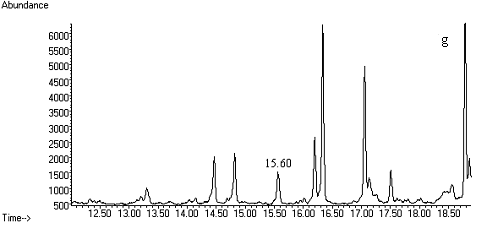
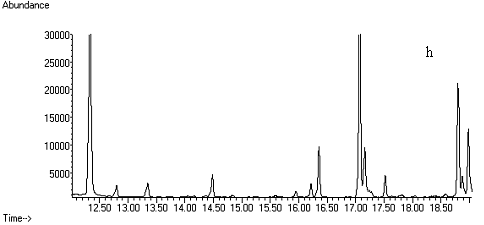
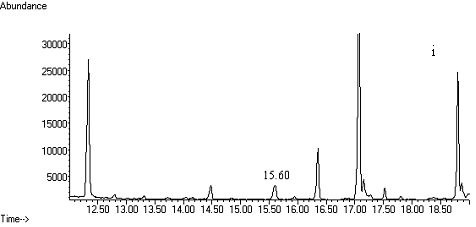
Fig. 2 Total ion chromatograms of TMS
derivative. (a) 5ng/mL clenbuterol standard; (b) kidney sample; (c) spiked kidney
containing 1mg/kg
clenbuterol; (d) lung sample; (e) spiked lung containing 1mg/kg clenbuterol; (f) liver sample; (g) spiked liver containing 1mg/kg clenbuterol; (h) meat sample; (i)
spiked meat containing 1mg/kg
clenbuterol.
The calculated limits of
detection are 1mg/kg
clenbuterol in the 5g tissue samples by a signal-to-noise ratio of 3:1. The limits of
detection of four kinds of tissue are lower than 0.4mg/ kg. They are 0.34mg/ kg in meat, 0.23mg/ kg in liver, 0.27mg/ kg in lung, and 0.22mg/ kg in kidney.
The recovery of clenbuterol was checked by adding clenbuterol standard
solution at three levels to untreated samples of four kinds of tissue. Blank samples from
the same tissue without fortification were treated and analysed at the same time with
spiked samples.
Complete recovery of the analytes through the extraction procedure was
achieved at both concentrations with very good coefficients of variation.
4. CONCLUSION
The method proposed involved extraction with perchloric acid and a simple clean-up by
extraction with isopropanol-ethyl acetate, and solid-phase extraction with SCX column. The
precision and accuracy of the method were found to be suitable for routine analysis of
clenbuterol. The method was successfully applied to the analysis of tissue samples after
administration of clenbuterol. The limit of detection is good enough to find clenbuterol
in tissues.
REFERENCES
[1] López-Erroz, C., Viñas, P., Cerdán, F.J et al. Talanta,
2000, 53, 47-53.
[2] Keskin, S., Özer, D., Temizer, A. Journal of Pharmaceutical and Biomedical
Analysis, 1998, 18, 639-644.
[3] Ventura, R., Damasceno, L., Farré, M et al. Analytica Chimica Acta, 2000, 418,
79-92.
[4] Amendola, L., Colamonici, C., Rossi, F et al. Journal of. Chromatography B, 2002, 773,
7-16.
[5] Xia, M. X., Liu, Y., Jiang, M., Chinese Journal of Analytical Chemistry. 2002, 30
(11): 1308-1311.
[6] Hernández-Carrasquilla, M. Analytica Chimica Acta, 2000, 408, 285-290.
[7] García, I., Sarabia, L., Ortiz, M. C., et al. Analytica Chimica Acta, 2004,
515, 55–63.
[8] Engelmann, M. D., Hinz, D., Wenclawiak, B. W. Analytical and Bioanalytical Chemistry,
2003, 375, 460-464.
[9] Whaites, L. X., Murby, E. J. Journal of Chromatography B, 1999, 728, 67–73.
[10] Guy, P. A., Savoy, M.-C., Stadler, R. H. Journal of Chromatography B, 1999, 736,
209-219.
[11] Williams, L. D., Churchwell, M. I., Doerge, D. R. Journal of
Chromatography B, 2004, 813, 35–45.
[12] Posyniak, A., Zmudzki, J., Niedzielska, J.
Analytica Chimica Acta, 2003, 483, 61-67.
[13] Sawaya W N, Lone K, Husain A et al. Food Control, 2000, 11, 1-5.
[14] Zhang, X. Z., Gan, Y. R., Zhao, F. N. Analytica Chimica Acta, 2003, 489, 95-101.
[15] Rashid, B.A., Kwasowski, P., Stevenson, D. Journal of Pharmaceutical and Biomedical
Analysis, 1999, 21, 635–639.
[16] Song, Y., Wang, D., Hu, Y et al. Journal of Pharmaceutical and Biomedical Analysis,
2003, 31, 311-319.
[17] Vela J., Yanes, E. G., Stalcup, A. M. Fresenius Journal of Analytical Chemistry,
2001, 369, 212–219.
[18] Zhou, H., Zhang, Zh., He, D et al.
Analytica Chimica Acta, 2004, 523, 237–242.
[19] Fente, C. A., Vázquez, B. I., Franco, C.,
Journal of Chromatography B, 1999, 726, 133-139.
[20] Traynor, I. M., Crooks, S. R. H., Bowers, J et al. Analytica Chimica Acta. 2003, 483,
187-191.
[21] Ramos, F., Cristino, A., Carrol, P et al. Analytica Chimica Acta, 2003, 483, 207-213.
[22] Koole, A., Bosman, J., Franke, J. P et al. Journal of Chromatography B, 1999, 726,
149-156.
[23] Bruins, C. H. P., Jeronimus-Stratingh, C. M., Ensing, K et al. Journal of
Chromatography A, 1999, 863, 115-122.
[24] Li, J. S., Qiu, Y. M., Wang, C. Analysis for Veterinary Residues, Shanghai Science
and Technology Publishing Company, Shanghai, 2002, p648~649.
[25] Zhou, L. M., Wan, B. H., Shang, Z. H. Hand Book of Modern Chemical Reagent-Sixth: Reagent for Instrumental Analysis, Beijing: Chemical
Industry Publishing, 1998, 238.
[26] Damasceno, L., Ventura, R., Ortuño, J et al. Journal of. Mass Spectrometry,
2000, 35, 1285-1294.
1刘芃岩 2王英锋
(1河北大学化学与环境科学学院,保定,071002,2首都师范大学化学系,北京,100037)
摘要 对猪组织中(肌肉、肝脏、肾和肺)克伦特罗的残留分析方法进行了研究,通过提取条件、固相萃取柱的选择以及不同衍生试剂的衍生条件的实验,确定了以高氯酸提取,用异丙醇-乙酸乙酯萃取和强阳离子(SCX)固相萃取两步净化,BSTFA(含1%TMCS)衍生后进行GC-SIMMS分析。在1mg/kg添加水平,信噪比3:1时,检出限小于0.4mg/kg,平均回收率均在73.4%以上,RSD小于10 %。在所选条件下,净化时只需一根SCX柱,从而降低了成本,节省了时间,操作步骤也得到简化,而且回收率、灵敏度满足了测定要求,可以作为动物组织中克伦特罗残留量的确证分析。
关键词 猪组织,克伦特罗,酸提取,SCX柱净化,测定