http://www.chemistrymag.org/cji/2005/07b071pe.htm |
Nov. 10, 2005 Vol.7 No.11 P.71 Copyright |
Magnetic-assisted micro-ozone applied to oxidize reactive blue dye simulated wastewater
Lu Xiuguo, Zhai Jian, Zhao Chunxia, Li Hui
(College of Chemistry and Environmental Science, HeBei University, Baoding 071002, China)
Received on Sep. 20, 2005.
Abstract The treatment of reactive
blue dye simulated wastewater by magnetic-assisted micro-ozone oxidation system has been
studied. The optimum conditions were investigated and the mechanism of degradation was
briefly discussed. The results showed that the removal rate of chromaticity achieved 97.8%
after oxidizing 30min without magnetic field. Comparatively, the removal rate of
chromaticity achieved 97.8% after oxidizing 24min under the action of magnetic field. The
result showed that the presence of magnetic field quickened the velocity of producing·OH
from O3 and improved the efficiency of oxidation.
Keywords magnetic field, micro-ozone, oxidation, reactive blue dye
1 INTRODUCTION
The aromatic hydrocarbon and the heterocyclic compound are mainly taken as a matrix in
the dying and printing wastewater. It has the colored groups and bases (for example -N=N-,
-N=O) and the polar groups and bases (for example -SO3Na, -OH, -NH2).
If the dye molecules contain many groups and bases (-SO3H, -COOH, –OH) which can form the hydrogen bond with water molecule like
reactive dyes and the neutral dyes and so on, then dye molecules can absolutely dissolve
in the wastewater; If the dye molecules contain no or little hydrophilic groups and bases
(-SO3H, -COOH, –OH), then dye molecules exist in the wastewater
in the form of suspended particulate. If dye molecules
contain few hydrophilic groups and bases but the molecular weight is very large or does
not contain the hydrophilic groups and bases, the dye
molecules usually exist in water as colloid form. Presently, the hydrophobic or water-fast
dye wastewater decoloration has basically been solved, but many hydrophilic or
water-soluble dye wastewater is difficultly decolored[1].
At present, the chroma and CODCr of dyeing wastewater is
usually removed by physical method, chemical method, or physical chemical method, and
biological method, but above methods have their insurmountable shortcomings. This article
takes reactive blue dye solution which is commonly used but difficultly biodegraded as the
breakdown products, micro-ozone is the oxidant and magnetic field is the sur-field. The
effect and efficiency which magnetic-assisted micro-ozone oxidation system degrades
reactive blue dye is explored, the coordinated oxidizing mechanism is preliminary
researched.
2 EXPERIMENTAL
2.1 Instruments and Reagent
Micro-ozone generator (Shanghai,China), KC-70C permanent magnetizer (Shanghai,China),
SXG-1B teslameter (Shanghai,China), UV-265 spectrophotometer (Japan).
Active blue dye solution, the soluble starch is the analytical purity,
the other are chemical purity.
2.2 Experimental procedures
2.2.1 Ascertain the dosage of ozone production
Appropriate amount of O3 was aerated into 500mL KI solution in a graduated
cylinder under normal temperature, for a certain time, the ration of oxidized solution (VKI)
was taken to titrate with the Na2S2O3, then the
productive quantity of ozone was calculated [2]
![]()
In the formula: Q and t respectively
expresses the ozone current capacity (L·min-1) and reaction time (min).
2.2.2 Ascertain the factors of the removal rate chroma of reactive blue dye simulative
wastewater
500mL simulative wastewater, regulates solution pH value, aerates the ration O3
under the normal temperature, reacts for a certain time, the absorbency of simulative
wastewater is determined, computes the decolorization rate.
![]()
In the formula: A0,A respectively expressed absorbency of reactive blue dye simulative
wastewater in the highest absorption peak place before and after treatment.
Magnetic-assisted micro-ozone oxidation experiment as the same to normal oxidation
experiment, the difference lies in which the reactive blue dye simulative wastewater in
the graduated cylinder is put in the magnetic field in the magnetic-assisted micro-ozone
oxidation experiment.
The factors which affect the removal rate of chroma are studied. These
factors include the time of aerating O3, pH value, intensity of magnetic field,
magnetization time.
2.2.3 The quality of raw wastewater and chromophore of reactive blue dye
The chromophore of reactive blue dye is ﹥C=N- group.
Table 1 The quality of raw wastewater
Concentration(mg·L-1) |
Chroma(times) |
pH value |
50 |
600 |
6 |
3 RESULTS AND DISCUSSION
3.1 The factors of the removal rate of chroma of reactive blue dye
3.1.1 The aeration time of ozone choice The pH value is 10, 500mL simulative
wastewater, the density of aerated ozone is 1.167mg·L-1·h-1,
at room temperature. The effect of ozone aeration time on the removal rate about chroma of
the simulative dyeing wastewater has been investigated.
From Figure1, it can be seen that the chroma of simulative reactive
blue dye is removed in a certain degree when ozone is aerated into this reaction system,
and the removal rate of chroma increase with the aeration time of ozone increasing. The
removal rate of chroma reached 97.8% when the aeration time of ozone is 30min. The removal
rate of chroma reached 99% when the aeration time of ozone is 35min. According to the
practical application, the best aeration time of ozone is 30min.
3.1.2 Reaction pH value choice 500mL simulative wastewater, the density of aerated
ozone is 1.167mg·L-1·h-1, the aeration time of ozone
is 30min, at room temperature. The effect of pH value of the solution (use thin vitriol or
sodium hydroxide to regulate) on the removal rate of chroma of the simulative dye
wastewater has been investigated.
From Figure2, it can be seen that the oxidization effect is best under
the alkalinity condition (namely pH 10). The reactive blue dye wastewater is a kind of
industrial wastewater which is difficultly degraded by biochemistry method. The general
chemistry method very difficultly oxidized it. Ozone directly attacks organic matter by
molecular form under acidity or neutral condition, moreover the molecular ozone response
has the greatly strengthened selectivity. It only reacts with the unsaturated aromatic
compound either the aliphatic compound or certain special groups and bases response. But
the reason which the removal rate of chroma enhanced under the alkalinity condition is
that ozone produces ·OH. It has stronger oxidation ability and is only inferior in
fluorin . Thus those difficultly biodegraded organic matter can be effectively degraded.
First, the passive groups( -NO2,-SO3-)of
dye molecules were substituted by ·OH, it made aromatic rings active. Then the
aromatic rings were decomposed and generated low fatty acid compounds. At last they were
turned into CO2 and H2O.
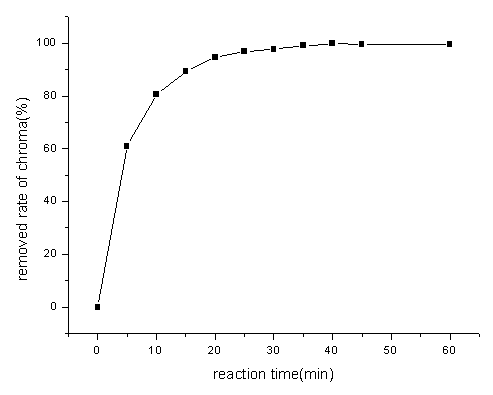
Fig1 Effect of the aeration time of O3
on colority removal
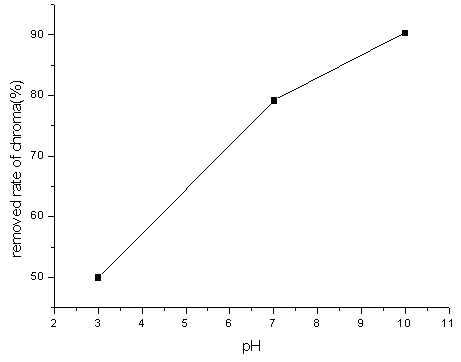
Fig2 Effect of pH value on colority removal
3.1.3 Sur-magnetic fields magnetizations time choice pH value is 10, 500mL
simulative wastewater, the density of aerated ozone is 1.167mg·L-1·h-1,
at room temperature. The effect of aeration time of ozone on the removal rate of chroma
under 374.1mT magnetic intensity is investigated.
The removal rate of chroma reached 97.8% needs 24min when the intensity
of sur-magnetic field is 374.1mT. It is 6min shorter than without magnetic field. But with
the magnetizations reaction time increasing, removal rate of chroma in intensity of
magnetic field being 374.1mT was close to which without magnetic field. The result showed
that the productive rate of hydroxyl radical was improved, but the productivity of hydroxyl radical was not
improved.
3.1.4 Intensity of sur-magnetic field choice The pH value is 10, 500mL simulative
wastewater, the density of aerated ozone is 1.167mg·L-1·h-1,
at room temperature, with the aeration time of ozone 30min.
The effect of intensity of sur-magnetic field on the removal rate of chroma is
investigated.
From Figure 4, it also can be seen that the removal rate of chroma by
different intensity of sur-magnetic field at the same reaction time was different. The
greater intensity of sur-magnetic field was, the more removal rate of chroma was. But with
the magnetizations reaction time increasing, the removal rates of chroma in different
intensity of magnetic field were close to each other. The result showed that the
sur-magnetic field enhanced productive rate of ·OH from micro-O3/OH-
system truly, but has not enhanced its productivity.
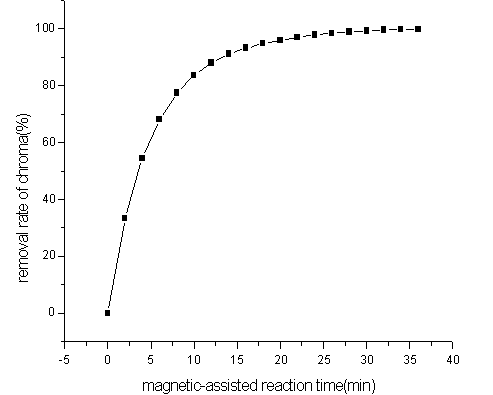
Fig3 Effect of magnetic-assisted reaction time on colority removal
Table2 The relation between A and reaction velocity
t/min |
A |
g/min-1 |
2 |
0.797 |
0.159 |
4 |
0.544 |
0.092 |
6 |
0.380 |
0.071 |
8 |
0.270 |
0.042 |
10 |
0.195 |
0.026 |
12 |
0.143 |
0.021 |
14 |
0.105 |
0.017 |
16 |
0.081 |
0.009 |
18 |
0.062 |
0.007 |
20 |
0.048 |
0.006 |
22 |
0.036 |
0.005 |
24 |
0.026 |
0.004 |
26 |
0.019 |
0.003 |
28 |
0.013 |
0.003 |
30 |
0.009 |
0.002 |
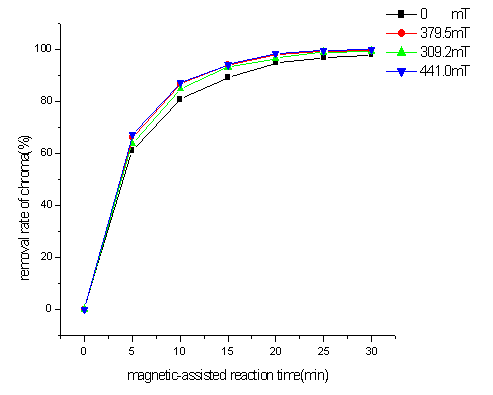
Fig4 Effect of intensity of magnetic field on colority removal
3.2 In magnetic field ozone oxidize active blue dye response dynamics When pH value
is 10, 500mL simulative wastewater, the density of aerated ozone is 1.167mg·L-1·h-1,
at room temperature, every two minutes take a sample until 40 minutes. Determine absorbency in the 600nm, the results are listed on Table 2.
According to data in the table, the relation between g and A
is simulated in first-order kinetics equation under the magnetic field condition, the
obtained correlation coefficient of linear equation above 0.99. It shows that the
decolority response which micro-ozone oxidizes reactive blue dye simulative wastewater
conforms to first-order reaction kinetics in the magnetic field [3].
3.3 Preliminarily reaction mechanisms research
It has been very early discovered that the magnetic field has the obvious response to
the free radical, free radical response is main response in the micro-O3/OH-
oxidation system, therefore extrapolates the magnetic field/micro-O3/OH- oxidation
system associated technology enhance the oxidation decomposed velocity of pollutant. It
does not consume extra electrical energy like the supersonic and ultraviolet ray (may
produce magnetic field by permanent magnet), is advantageous for application.
Under not sur-magnetic field condition, a magnetic field which being
from the molecular interior (is called partial magnetic field) could provide the magnetic
moment for the change of the spinning condition of the free radical. Under sur-magnetic
field condition, the Larmor spinning velocity of the spinning vector of the free radical
can be changed. The difference of the spinning velocity of two spinning vectors (Dw) can be expressed by the formula
![]()
![]()
In the formula, Dg =|g1-g2|, g1 g2
respectively notes the g value of two free radicals alone electron, g value is related
with the system nature, mB
is a Bohr's magneton, h is the Planck constant, H0 is the intensity of
sur-magnetic field, a1, a2 are ultra fine coupling constants of two
free radicals, I is the nuclear spin quantum number. The transformation speed which the
two spinning vectors of free radicals from S state to T0 state is decided by Dw value. According to above formula,
the above transformation speed can increase with Dg or Ho enhancing[4]. Speaking of the reactive blue dye
simulative wastewater, Dg
is a definite value in the microscopic structure, therefore the difference of the Larmor
spinning velocity (Dw) is
only decided by the intensity of macroscopic sur-magnetic field. According to Dg mechanism, the bigger H0,
the shorter transformation time and the quicker transformation speed from S state to T0
state will be. Namely the more little-singlet state total,
the smaller possibility of re-combination and the quicker rate of chemical reaction will
be. This is the degeneration process mechanism of magnetic-assisted micro-O3/OH-
system.
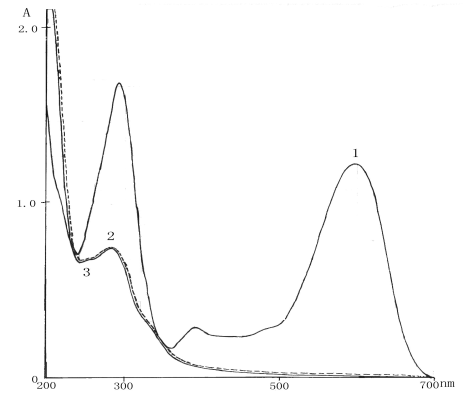
Fig5 Sample ultraviolet visible spectrum before and after treatment
1-before treatment, the ultraviolet visible spectrum of sample
2-after oxidation under magnetic field condition, the ultraviolet visible spectrum of
sample
3- after oxidation without magnetic field condition, the ultraviolet visible spectrum of
sample
4 CONCLUSIONS
4.1 Ultraviolet visible spectrum of the sample before and after treatment respectively
Before treatment, the sample has the obvious absorption peaks in the ultraviolet
visible range. After magnetic-assisted oxidation treatment, there are not absorption peaks
in the visible light area. It shows that the color groups and bases are completely
destroyed. At the same time the 300nm scan line also obviously reduced. It shows that the
benzene ring is partially oxidized by the hydroxyl free radical and produced chain framework. Curve 2 (reaction time was 24min) dashed lines denotes
the ultraviolet visible spectrum of sample which is oxidized by magnetic-assisted
oxidation system. Curve 3 (reaction time was 30min) solid line denotes the ultraviolet
visible spectrum of sample which is oxidized by only oxidation system. The two ultraviolet
visible spectrums are extremely approximate (reaction time is obviously different, dashed
line is add-on shows difference). From Fig5 we can see that the productivity of ·OH
under sur-magnetic field was the same to no sur-magnetic field. It shows that the
sur-magnetic field did not enhance the productivity of ·OH.
4.2 Conclusions
1. When 500mL simulative wastewater, pH value is 10, the density of aerated ozone is
1.167mg·L-1·h-1, the intensity of magnetic field is
374.1mT, the removal rate of chromaticity achieved 97.8% after oxidizing 30min without
magnetic field. Comparatively, the removed rate of chromaticity achieved 97.8% after
oxidizing 24min under the presence of magnetic field.
2. According to first-order reaction kinetics, the relation between g and A is fit in first-order kinetics
equation under the magnetic field condition, the obtained correlation coefficient of
linear equation above 0.99. It shows that the response fits in first-order kinetics, the
kinetics equation is g=0.19246A.
3. The sur-magnetic field enhanced ·OH productive rate of micro-O3/OH-
system, but has not enhanced its productivity.
[1] Zheng Jilu, Fan Juan, Ruan Fuchang. Techniques and equipment for environmental pollution control, 2000, 1 (5): 29-35.
[2] Zheng Yunzhong. the measurement of the ozone density, synthesize the material aging and applied 1990,000 (002): 37-41
[3] Jiang Huanwei, Wang Yu , Li Fengkai et al. Journal of East China University of Science and Technology ( natural science version), 2003, 29 (2): 166-169.
[4] WangGuoquan, WuZhongyun, magnetic chemistry and medicine. BeiJing: Weapon Industry Press,1997,21-23.
磁强化微臭氧氧化体系对活性蓝染料废水脱色的实验研究
(河北大学化学与环境科学学院环境科学系 河北保定 071002)
摘要 本文利用磁强化微臭氧(O3) 氧化法协同体系对活性蓝染料废水进行了脱色处理实验研究,筛选出了最佳实验条件, 并对降解机理进行了简要讨论。实验结果表明,无磁场条件下, 通入适量O3,反应30 min后,色度去除率达97.8%。外加磁场作用,反应24 min后,色度去除率即可达到97.8 %。磁场的存在强化了O3生成·OH的速率, 进一步提高氧化的效率。
关键词 磁场 微臭氧 氧化 活性蓝染料