http://www.chemistrymag.org/cji/2005/07b072pe.htm |
Nov. 10, 2005 Vol.7 No.11 P.72 Copyright |
Shan
Jinhuan , Liu Hongmei , Huo Shuying , Shen Shigang , Sun Hanwen
(Key Laboratory of Analytical Science and Technology of Hebei Province, College of
Chemistry and Environmental Science, Hebei University, Baoding 071002, China)
Abstract The kinetics of oxidation of 1,2-butyleneglycol (a-BG) by dihydroxydiperiodatonickelate ( IV) were studied spectrophotometrically between 298.2K and 313.2K in alkaline medium. The reaction rate showed first order dependence on dihydroxydiperiodatonickelate ( IV) and 1<nap<2 order in a-BG. It was found that the pseudo-first order rate constant kobs increased with increasing concentration of OH- and decreasing concentration of IO4-. There is a negative salt effect and no free radical was detected. In view of this, the dihydroxydiperiodatonickelate ( IV) species is assumed to be the
existent form. A plausible mechanism involving a two-electron transfer is proposed and the rate equations derived from the mechanism can explain all of the experimental results. The activation parameters, as well as the rate constants of the rate-determining step were calculated.Keywords dihydroxydiperiodatonickelate( IV) , 1,2–butyleneglycol, redox reaction, kinetics and mechanism In general, transition metal Ni only consistsin stabilizaed Ni(II), however, if there exist some ligands,the higher oxidation state of nickel, such as Ni(III), Ni(IV) can also exist stably, they can form complexes not only with iodic acid[1], molybdic acid[2] and telluric acid[3] and so on, but also with organic substance, for example: [NiⅣ(L1)]2+ and [NiIV(L1) 2]2+(where H2L1= 3,14-dimethyl -4,7,10,13-tetraazahexadeca-3,13 -diene- 2,15-dione dioxime and HL2 = 6-amino-3-methyl-3-en-2-one oxime) [4].
The use of Ni( IV) as an oxidizing agent is well known in the investigation of some organic compounds such as tetrahydrofurfuryl alcohol[5], ethylene diamine [6], glycollic acids[7] etc .Since Ni( IV) is in the higher oxidation state and the reaction is complicated in this system, it is of significance to have a further study on this kind of reaction system. In this paper, the kinetics and mechanism of oxidation of 1,2-butyleneglycol by dihydroxydiperiodatonickelate ( IV) is put forward, also, from the k and activation parameters got from the experiment, the microcosmic structure of transition state compound, has been studied for the sake of understanding the microcosmic information of the reaction.
1. EXPERIMENTAL
1.1 Materials
All the reagents used were of A.R. grade. All solutions were prepared with redistilled
water. A solution of [Ni(OH)2(H2IO6)2]4-
(DPN) was prepared and standardized by the method reported earlier[8]. Its UV spectrum was found to be consistent with that
reported in the literature. The concentration of DPN was derived from its absorption at l = 410nm (e
1.2 Kinetics measurements and product analysis
All kinetics measurements were carried out under pseudo-first order conditions. A solution (2 mL) containing Ni(IV), OH-, IO4- with ionic strength m and a a-BG solution (2mL) of an appropriate concentration were transferred separately to the upper and lower branch tubes of a type two-cell reactor. After thermal equilibration at the desired temperature in a thermobath, the two solutions were mixed well and immediately transferred in a 1cm rectangular cell quartz in a constant temperature cell-holder (±0.1oC). The reaction process was monitored automatically by recording the disappearance of Ni(IV) with time (t) at 410 nm with a UV-8500 spectrophotometer. All other species did not absorb significantly at this wavelength.
After completion of the reaction, the oxidation product was identified as an aldehyde alcohols which was precipitated as 2,4-dinitrophenyldrazone derivative. By gravimetric analysis, it is found that one mole of a-BG consumed one mole Ni(IV) .
2. RESULTS AND DISCUSSION
2.1 Evaluation of pseudo-first order rate constants
Under the conditions of [a-BG]0>>[ Ni(IV)]0, the plots of ln(At-A∞) versus time were straight lines, indicating the reaction is first order with respect to [Ni(IV)], where At and A∞ are the absorbance at time t and at infinite time ,respectively. The pseudo-first-order rate constants kobs were calculated by the method of least squares (r≥0.999). Generally, 8-10 At values within three times the half-lives were used to calculate kobs. The kobs values were the average values of at least three independent experiments.
2.2 Rate dependence on [a-BG]
At fixed concentration of Ni(IV), OH-, IO4-, ionic strength m, the values of k obs were determined at different temperatures and a-BG concentrations. The kobs values was found to increase with increasing concentration of a-BG at all temperatures with an apparent order of 1<nap<2. The plots of [a-BG]/kobs versus 1/[a-BG] were straight lines with a positive intercept. (Table 1). Table 1 103 kobs varying with different concentrations of a-BG at different temperatures
| 102[a-BG] (mol/L) | 0.50 |
0.60 |
0.80 |
1.30 |
2.50 |
nap |
r |
a |
r1 |
|
T(K) |
298.2 | 2.569 |
3.341 |
5.395 |
10.19 |
25.96 |
1.43 |
0.999 |
0.7548 |
0.995 |
| 303.2 | 4.199 |
5.505 |
8.227 |
15.66 |
37.99 |
1.34 |
0.999 |
0.5642 |
0.992 |
|
| 308.2 | 7.456 |
9.457 |
14.02 |
24.91 |
52.65 |
1.21 |
0.999 |
0.4230 |
0.998 |
|
| 313.2 | 12.41 |
15.13 |
21.54 |
37.28 |
76.97 |
1.17 |
0.999 |
0.3002 |
0.996 |
|
2.3 Rate dependence on [IO4-]
At fixed concentration of Ni(IV), OH-, a-BG、ionic strength m and temperature, the values of kobs were determined.The value of kobs decreased with the increasing concentration of IO4- .The plots of 1/kobs versus [IO4-] were straight lines with a positive intercept.(Table 2 and Fig.1)
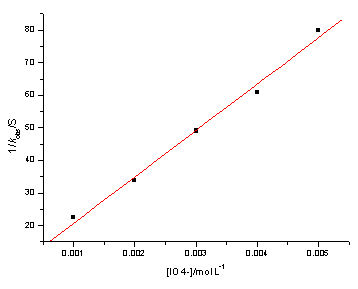 |
Fig.1 1/kobs
versus [[IO4-],[Ni(IV) ]=8.04×10-6mol/L; T= 303.2 K; [a-BG]=2.00×10-2 mol/L; [OH-]=2.00×10-2 mol/L; m=0.12 mol/L |
Table 2 Rate dependence on [IO4-]
103[IO4-] (mol/L) |
1.00 |
2.00 |
3.00 |
4.00 |
5.00 |
102kobs (s-1) |
4.459 |
2.964 |
2.040 |
1.643 |
1.250 |
[Ni(IV) ]=8.04×10-6mol/L; T= 303.2 K; [
α-BG]=2.00×10-2 mol/L; [OH-]=2.00×10-2 mol/L; m=0.12 mol/L 2.4 Rate dependence on [OH-] and ionic strength mAt fixed concentration of Ni(IV), IO4-, a-BG, ionic strength m and temperature, the value of kobs increased with the increase of concentration of OH-. The order with respect to OH- was found to be fractional. The plots of 1/kobs versus f(OH)/[OH-] were straight lines (r≥0.990) (Table3).The value of kobs decreased with the addition of KNO3 solution (Table4), which indicates that there was a negative salt effect, which was consistent with the common regulation of the kinetics[9].
Table 3 Rate dependence on [OH-]
102[OH-] (mol/L) |
2.00 |
4.00 |
6.00 |
8.00 |
10.00 |
102kobs (s-1) |
1.079 |
1.544 |
1.980 |
2.533 |
2.945 |
[
Ni(IV) ]=8.04×10-6mol/L; T= 303.2 K; [a-BG]=1.00×10-2 mol/L; [IO4-]=2.00×10-3 mol/L; m=0.12 mol/LTable 4 Rate dependence on m
| m mol/L | 0.07 |
0.12 |
0.17 |
0.22 |
0.27 |
102kobs (s-1) |
4.046 |
3.046 |
2.602 |
2.106 |
1.736 |
[Ni(IV) ]=8.04×10-6mol/L; T= 303.2 K; [
a-BG]=1.00×10-2 mol/L; [IO4-]=2.00×10-3 mol/L; [OH-]=2.00×10-2 mol/L3 DISCUSSION OF THE REACTION MECHANISM
In an alkaline medium, the electric dissociation equilibrium of periodate was given
earlier [10] (here pKw=14)
IO4- + OH- + H2O
IO4- + 2OH-
The distribution of all species of periodate in aqueous alkaline solution can be calculated from the equilibriums (1)-(3). The dimer H2I2O104- and IO4- species can be neglected, the main iodic acid species was H3IO62- and H2IO63-. According to the literature, the main existent form of oxidant was [Ni( OH) 2( H2IO6) 2]4-over the experimental concentration range of OH-.
The addition of acrylonitrile or acrylamide to the reaction mixture under a nitrogen atmosphere neither changed the reaction rate nor initiated any polymerization, showing no free radical were involved in the reaction[11]. It is thought that under the employed conditions a type one-step, two-electron transfer mechanism is in operation.
According to the above experimental fact, the following reaction mechanism is proposed
[Ni( OH) 2(H2IO6) 2]4- + OH-
[Ni( OH) 2(HIO6) ]2-+[a-BG]
[Ni( OH) 2(HIO6) ]2-·[a-BG]+[ a-BG]
complex
complex
Reaction (4) and (5) are in dissociation and coordination equilibria, the reaction rates of which are generally fast, reaction (6) belongs to an electron-transfer reaction, the reaction rate of which are generally slow, hence reaction (6) is the rate-determining step.
-d[Ni(IV)]t/dt = k[Adduct] [a-BG]
where [Ni(IV)]t stands for any form of Ni(IV)complex which exists in the equilibrium.
-d[Ni(IV)]t/dt =
= kobs[Ni(IV)]t
![]()
Neglecting the concentration of ligand dissociated from Ni( IV) and the species of periodate other than H2IO63- and H3IO62-, here:
[IO4-]ex ≌ [H3IO62-]+[H2IO63-] (12)
Equations ( 13) and ( 14) can be obtained from ( 2) , ( 3) , ( 12) :
![]() (15)
(15)
![]() (16)
(16)
The activation energy and the thermodynamic parameters were evaluated by the method given previously.[12](Table 5 and Fig. 2)
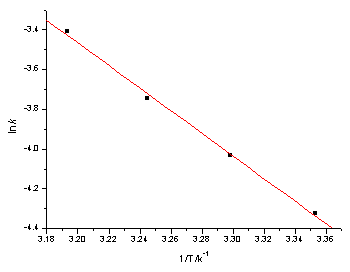 |
Fig.2 ln k
versus 1/T [Ni(IV) ]=8.04×10-6 mol/L; [OH-]=2.00×10-2 mol/L; m=0.12 mol/L; [IO4-]=2.00×10-3 mol/L |
Table 5 Rate constants (k) and activation parameters of rate-determining step(298.2K)
k (L mol-1 s-1) |
T (K) |
Activation parameters | |||||
298.2 |
303.2 |
308.2 |
313.2 |
Ea (KJ·mol-1) |
DH# (KJ·mol-1) |
D S#(J·mol-1K-1 ) |
|
| a-BG | 1.325 |
1.772 |
2.364 |
3.331 |
47.60 |
45.12 |
-91.42 |
DEA* |
11.90 |
15.49 |
21.16 |
29.74 |
46.28 |
43.81 |
-113.22 |
The plot of ln k vs -1/T has the following intercept (a),slope(b),and relative coefficient(r):
a-BG a = 19.46 , b = -5.72×103, r = 0.998 ; DEA a = 21.46 , b = -5.57×103 , r = 0.998* based on our previous work 4. CONCLUTION
Contrast to the previous experiment, it can be concluded that the observed rate constants of the rate -determining step for diethanolamine are larger than those for 1,2-butyleneglycol. Because the -NH2 embody the stronger alkalescence than -OH, according to coordination chemistry[13] ,we know the stability of the coordination compound increased with the increase of the alkalescence. In addition, the 1,2-butyleneglycol embody a structure of ethyl, which has the special steric hindrance. So the formation of intermediate adduct between Ni(IV) complex and diethanolamine is more stable than that of 1,2-butyleneglycol, which leads up to the observed rate of the rate -determining step of diethanolamine faster than 1,2-butyleneglycol , this is consistent with the result from the experiment.
REFERENCES
[1] Siddiqui M A A, Khan J A, Kandlikar S. Indian J. Chem, 1993, 32A: 174-176
[2] Dunne S J, Burns R C. And Lawrance G A. Aust.J.Chem. 1992, 45: 1943-1952
[3] Mukherjee H G, Dutta S.K. and Sen S. Indian J. Chem. 1996, 73: 598-599
[4] Amitava D, Santanu B and Pradyot B. Polyhedron, 1998, 17:2313-2319
[5] Li Z T, Wang F L, Wang A Z. Int. J. Chem. Kinetics, 1992, 24: 933-941
[6] Li Z T, Chang Q, Li B W et al. Chinese Research in Chinese Universities (GaodengxuexiaoHuaxuexuebao), 2000, 21( 5) : 747-751.
[7] Chandraiah U, Murthy C P, Kandlikar S. Indian J.
Chem,1989,28(A):162-163.
[8] Shan J H,Wei H.Y,Wang L.L et al. Indian J. Chem,2001,40 (A): 865-869.
[9] Jin J J. Kinetics Principle of Chemical Reaction in Liquid Phase, The frist edition,
Science Technique Press, Shanghai, 1984, 186.
[10] Aveston J. J. Chem.Soc. ( A) , 1969: 273-275.
[11] Chandraiah U,Jaffar A.K, Murthy C P et al. Indian J of Chem, 1987, 26A: 481-484.
[12] Shan J H, Liu T Y. Acta Chem Sin., 1994,52: 1140-1144
[13]Fred B, Ronald C. J. Coordination Chemistry. Beijing University Press, 1982,305-307
碱性介质中二过(碘酸根)合镍(IV)酸根氧化b
-丙二醇的反应动力学及机理单金缓 刘红妹 霍树营 申世刚 孙汉文
( 河北省分析科学与技术重点实验室, 河北大学化学与环境科学学院,保定 071002)
摘要 用分光光度法在298.2-313.2K区间研究了碱性介质中二羟基二(高碘酸根)合镍(IV)酸根(DPN)氧化1,2-丁二醇(a-BG)的反应动力学及机理。结果表明:反应对DPN为准一级,对a-BG 为1<nap<2级;在保持准一级条件([a-BG]0 >> [DPN]0)下,表观速率常数随着[OH-]的增加而增大,随着[IO4-]的增加而减小;存在负盐效应且没有自由基被检测出来。由此,作者认为[Ni( OH) 2( H2IO6) 2]4-是存在形式,并且提出了一种双电子传递机理,由假设反应机理推出的速率方程能很好地解释全部实验现象,进一步求得速控步的速率常数和活化参数.
关键词 二羟基二(高碘酸根)合镍(IV)酸根 1,2-丁二醇 氧化还原反应 动力学及机理