http://www.chemistrymag.org/cji/2005/07b075pe.htm |
Nov. 25, 2005 Vol.7 No.11 P.75 Copyright |
Rapid determination of nitrate in fresh vegetables by ion chromatography
Liang Shuxuan, Chen Qiu Sheng, Liu Zhanfeng,
Sun Hanwen
(Key Laboratory of Analytical Science and Technology of Hebei Province, College of
Chemistry and Environmental Science, Hebei University, Baoding 071002, China)
Received on Aug. 23, 2005; Supported by Natural Science Foundation of Hebei University (Grant No. 026)
Abstract A simple, rapid ion
chromatography with suppressed conductivity detection to detect nitrate in vegetables was
developed. Extraction methods and the composition of eluent
were studied. Ultrasonic wave extraction was chosen. The eluent used was composed of 5.8
mmol/L sodium carbonate -4.8 mmol/L sodium bicarbonate and the eluent flow-rate was
1.5ml/min. Under the optimum conditions, the nitrate and other ions were
separated effectively. The relative standard deviation was 0.98% for 6 parallel tests.
Recoveries at three different added levels were 107.3%, 94.9% and 96.7% respectively. The
limit of detection was 0.053mg/L under the S/N ratio 3:1. The nitrate concentrations in
fresh cucumber, cole, lettuce, tomato and celery from local markets were determined by
this method. When compared with National Standard Method of P. R. China, it was revealed
that the accuracy and sensitivity were suitable for the
determination requirements. The method could be applied to the
routine analysis of nitrate in fresh vegetables.
Keywords Nitrate; Vegetables; Ion chromatography; Ultrasonic extraction
Nitrate and nitrite are ubiquitous within
environmental, food, industrial and physiological systems. The nitrate anion is an
important analyte for the environmental problem and for human health, whose detection and
quantification is essential. Although nitrate is more stable and less toxic than nitrite,
nitrate can readily be converted to nitrite by microbial reduction in food products and
nitrite which reacts with secondary amines in the stomach is induced to the carcinogenic
nitrosamine. In addition, nitrite is also known to cause methemoglobinemia (oxygen
deficiency) in infants [1, 2]. A risk assessment has been made on nitrate,
nitrite and N-nitroso compounds encountered in the human diet. Vegetables constitute a
major source of nitrate providing over 85% of the average daily human dietary intake
[3].
Due to these toxic effects, it is essential to have a sensitive, rapid,
low cost and reliable technique to detect these ions. Several methods have been reported
for the determination of nitrate in foods and biological materials, including
spectrophotometric methods [4 -7],high-performance
liquid chromatography (HPLC) [8,9], capillary electrophoresis (CE) [10,11],ion chromatography (IC) [12-15] . The spectrophotometric
method is based on a diazotization of various aromatic amines with nitrite in acidic
medium and on a subsequent coupling of the diazonium ions with N- (1-naphthyl)
ethylenediamine. This method has traditionally been used, but it is subjected to
interference from other species present and time consuming. Besides, a heavy investment in
reagents is necessary for each sample being determined. Recently ion chromatography is
gaining wide acceptance as a useful method for the determination of anions in
environmental samples with simple matrix.
In this study, a fast ion chromatography with a supersonic extraction
technique was examined for the fast and sensitive determination of nitrate in vegetable
samples.
1. EXPERIMENTAL
1.1 Instrumentation
The IC system consisted of: (1) a CIC-100 ion chromatograph with a suppressed
conductivity detector (Shenghan Chromatograph Technology Co. Ltd., Qingdao, China); (2) a
sample injector with a 100 mL
sample loop; (3) a YSA8 analytical column (250mm×4.6mm i.d.) (Research Institute of
Nuclear Industry, China); (4) a HW-2000 chromatography workstation (Qianpu Software
Co.Ltd., Zhejiang, China). Ultrasonic cleaner (Ultrasonic Instrument Co.Ltd., Kunshan,
China) was used to extract inorganic anion in vegetables. Sep-PakC18 (Waters, USA)
cartridge was used to remove organic interference. UV-265 spectrophotometer (Shimazu,
Japan) was employed for the determination of nitrate to compare to IC.
1.2 Reagents
The water for the preparation of all solutions was made by XGJ-30 ultra-pure water system
(Yongcheng Water Treatment Technology Co.Ltd., Beijing, China). Sodium
nitrate, sodium carbonate and sodium bicarbonate were obtained from Tianjin Chemicals
Co.Ltd., (Tianjin, China). All the reagents were analytical grade unless indicated
otherwise specified.
The fresh vegetables samples were purchased from the local markets.
1.3 Sample preparation
The fresh vegetable samples were carefully rinsed with tap water, distilled water and
deionized water in turn, then chopped and homogenized in a blender for 1 min. 20 g of each
homogenate was transferred to a 250 ml conical flask with 5ml ammonium chloride buffer, 90
ml deionized water and 1 g active carbon, and shook for 10 min in ultrasonic wave at 70ºC, then cooled
to room temperature and transferred to a 250 ml volumetric flask. To each volumetric
flask, 2 ml potassium ferrocyanide and 2 ml zinc acetate solution were added. The flasks
were then filled to volume with deionized water and filtered through a 0.45 mm cellulose acetate filter disk and a
Sep-PakC18 in order. Finally the filtrate was collected for IC analysis.
1.4 Experimental procedure
Nitrate was separated from the other ions and detected by isocratic conditions with an
YSA8 analytical column. When injections of standard solution give reproducible retention
times and peak areas, each sample solution is then injected, in diluted form if necessary,
into the IC apparatus in an aliquot of 100 mL for analysis.
2. RESULTS AND DISCUSSION
2.1 Selection of extraction methods
Three extraction methods including directly hot water extraction, shaker extraction and
ultrasonic wave extraction were applied to the same sample of cucumber for 3 times
respectively in order to compare the efficiency among them. The effect of different
extraction methods was shown in Table 1.
It was revealed that the NO3- was extracted most
effectively from vegetable by ultrasonic wave extraction. In addition, the shaker and hot
water extraction were time-consuming, and they were also very difficult to handle a large
number of samples efficiently. Ultrasonic wave method has advantages in many ways.
Therefore, the ultrasonic wave was an efficient method to transfer the soluble inorganic
anions from the vegetable samples to water rapidly. So in our experiment, all samples were
dealt with the ultrasonic wave method.
Table 1 The results of three extraction methods
Extraction method |
Content (mg/kg)* |
Recovery (%) |
Hot water extraction |
148.6 |
63.5 |
Shaker extraction |
151.5 |
73.1 |
Ultrasonic wave extraction |
163.9 |
98.0 |
* Average of three determinations for each sample.
2.2 Selection of eluent compositions
In routine single column IC, especially when a suppressed conductivity detector is used,
the retention time, resolution and responding sensitivity were rested with the ionic
strength, flow rate and pH of the eluent. The ionic strength and pH of the eluent were
adjusted by varying the concentrations of sodium carbonate and sodium bicarbonate.
In this work, it was shown that mixed solution of 5.8 mmol/L sodium
carbonate -4.8 mmol/L sodium bicarbonate was a
suitable eluent. With this eluent, NO3- can be separated
effectively from other ions. The standard chromatogram was shown in Fig. 1.
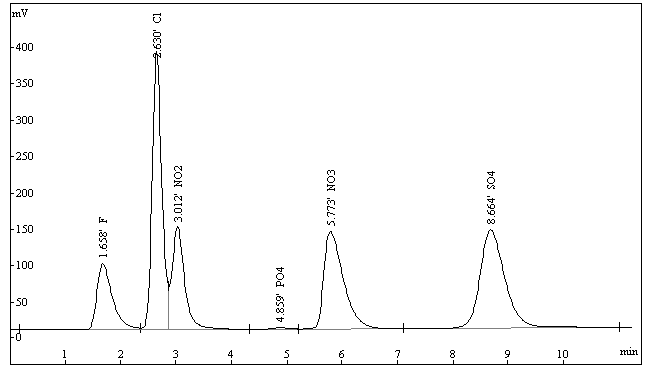
Fig. 1 The standard Chromatogram of six kinds of ion
2.3 Linear range and detection limit
of the method
A calibration graph was constructed by plotting the peak areas against the concentrations
of the standard injection for nitrate. Nitrate was injected in triplicate injections at 5
levels ranging between 0.3mg/ml
to 30mg/ml. The limit of
detection was 0.053 mg/L under the S/N ratio 3:1. The linear equation and the detection
limit of nitrate are listed in Table 2.
It is obvious from the data presented in Table 2 that the peak area and
the concentrations of the detected sample exhibited well linear relation. And the
calibration graph exhibited wide linear concentrations ranges with correlation coefficient
0.9996.
Table 2 Linear equations and detection limits
Composition |
linear equation |
correlation coefficient |
detection
limit |
P(N=6) |
Nitrate |
Y=-147912.738+3.413e+005X |
0.9996 |
0.053 |
﹤0.0001 |
Y-peak area; X-the content of composition (mg/ml)
2.4 Repeatability and recoveries
The degree of agreement among individual test results was determined and expressed as RSDs
(relative standard deviations). Sample solutions of cucumber were repeatedly determined
for successive six times. The RSD obtained was 0.98%. The method showed the retention time
unchanged after 60 injections for vegetable samples.
Spike recovery experiments were performed to determine the percent
recovery of added standards. Known amounts of nitrate standards were added to each of the
vegetable samples and allowed to be absorbed into the vegetable samples for 10 min. The
amounts of nitrate were then determined following the same extraction and separation
processes. The results were listed in Table 3.
Table 3 Recoveries of the nitrate
Original content(mg/kg) |
Added content(mg/kg) |
Total content found(mg/kg) |
Mean
recovery |
RSD |
134.5 |
10.0 |
144.5,140.9,150.3 |
107.3 |
0.33 |
15.0 |
149.5,155.1,141.6 |
94.9 |
0.46 |
|
20.0 |
153.5,146.3,161.7 |
96.7 |
0.50 |
2.5 Practical analysis of various
vegetable samples
The proposed method was applied to determine nitrate in various vegetable samples
(purchased from the local markets). The chromatogram of celery sample was given in Figure
2. To validate the present IC method, the vegetable samples were also determined by the
spectroscopic method after Cd-catalysed reduction to NO2 according to National
Standard Method of P. R. China (GB/T 5009.33-2003) [4]. The comparison of the
results was listed in Table 4. The results showed that the
accuracy and sensitivity of the proposed IC method were suitable for the determination
requirements.
Table 4 Nitrate concentrations in vegetable samples* (mg/kg)
Sample |
IC method |
GB method |
Relative Error (%) |
Cucumber |
134.5 |
163.9 |
-17.9 |
Cole |
4547.5 |
4555 |
-0.2 |
Lettuce |
1067.6 |
1044 |
+2.3 |
Tomato |
4.637 |
4.577 |
+1.3 |
Celery |
5213.8 |
5198.3 |
+0.3 |
*Three determinations for each sample.
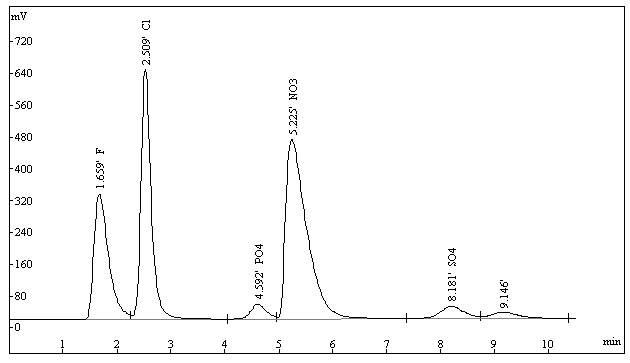
Fig.2 Chromatogram of celery sample
3. CONCLUSION
The proposed system is simple, cheap, and allows rapid and sensitive determination of
nitrate in vegetable samples. It is unlike the spectroscopic method which is reagent and
time consuming.
REFERENCES
[1] F. N. Assubaie. Pakistan Journal of Biological Sciences, 2004,7 (4): 508-513
[2] Sharat D. Gangolli, Piet A. van den Brandt, Victor J. Feron et al. EJP Environmental
Toxicology and Pharmacology, 1994, 292: 1-38.
[3] Matthew J. Moorcroft, Jams Dvais, Richard G.. Compton. Talanta, 2001, 54: 785-803.
[4] National Standard of the People's Republic of China. GB/T 5009.33. Beijing: China
Standard Press, 2003.
[5] Wang G F, Kiyoshi Horita, Masatada Satake. Microchemical Journal, 1998, 58: 162-174.
[6] A. Kazemzadeh, Ali A Ensafi. Microchemical Journal, 2001, 69: 159-166.
[7] Raquel Andrade, Claudia O. Viana, Silvane G. Guadagnin et al. Food Chemistry, 2003,
80: 597-602.
[8] Li H, Cynthia J. Meininger, Wu G Y. Journal of Chromatography Part B, 2000, 746:
199-207.
[9] Vera Jedlichova, Zoltan Paluch, Stefan Alusik. Journal of Chromatography B, 2002, 780:
193-197.
[10] M. Jimidar, C. Hartmann, N. Cousement et al. Journal of Chromatography A, 1995, 706:
479-492.
[11] Nevin Oztekin, M. Said Nutku, F. Bedia Erim. Food Chemistry, 2002, 76: 103-106.
[12] Daniel C. Siu, Alan Henshall. Journal of Chromatography A, 1998, 804: 157-160.
[13] Murad I.H. Helaleh, Takashi Korenaga. Journal of Chromatography B, 744: 433-437.
[14] Yang M, Li G J, Zhong G H, et al. Physical Testing and Chemical
Analysis: CHEM. ANAL (Lihua Jianyan-Huaxue Fence), 2005, 41: 412-414.
[15] Zhou M S, Guo D L. Chinese Journal of Analytical Chemistry (Fenxi Huaxue), 2000, 28:
1056-1056.
梁淑轩 陈秋生 刘占峰 孙汉文
(河北省分析化学重点实验室,河北大学化学与环境科学学院,保定, 071002)
摘要 介绍一种简单、低成本的测定新鲜蔬菜中硝酸盐含量的离子色谱-电导检测方法。对提取方法和淋洗液的配比进行了选择研究,应用简便高效的超声提取技术,以5.8 mmol/L NaCO3 - 4.8 mmol/L NaHCO3 为淋洗液,流速为1.5mL/min,硝酸盐和相邻离子能得到良好的分离。相对标准偏差0.98%(n=6);三个不同添加水平的平均回收率分别为107.3%, 94.9%,96.7%;检出限为0.053mg/L(S/N=3)。并应用此方法对当地农贸市场的黄瓜、油菜、莴苣、番茄、芹菜等新鲜蔬菜的硝酸盐含量进行了检测,同时用国标方法做了对比,结果显示,这种方法的准确度和灵敏度均达到测定要求,可以用于新鲜蔬菜样品中硝酸盐含量的日常检测。
关键词 硝酸盐 蔬菜 离子色谱 超声提取