http://www.chemistrymag.org/cji/2005/07b077pe.htm |
Nov. 25, 2005 Vol.7 No.11 P.77 Copyright |
(College of Chemistry and Environmental Science, Hebei University, Baoding 071002, China)
Received on Oct. 11, 2005.
Abstract The kinetics and mechanism of iridium(III) catalyzed oxidation of methanol by cerium(IV) in sulfuric acid media has been investigated by titrimetric technique in the temperature range of 25 - 40
ºC. The reaction order of [Ce(IV)] was found to be unity, and that in both methanol and iridium(III) to be positive fractional. It was found that the pseudo first order ([CH3OH]>>[Ce(IV)]) rate constant kobs increases with the increase of [H+], but decreases with the increase of [Keywords Iridium(III) ion, cerium(IV) ion, methanol, catalyst, kinetics and mechanism
1 INTRODUCTION
Transition metals in a higher oxidation state generally can be
stabilized by chelation with suitable complex agent. Metal complexes such as
diperiodatocuprate(III)[1], dihydroxydiperiodatonikelate(IV)[2] and
Ce(IV) ions[3] are good oxidants in a medium with an appropriate conditions.
However, our preliminary observations indicate that oxidation of some organic compounds by
Ce(IV) in aqueous sulfuric acid is kinetically sluggish, the process can be efficiently
catalyzed by various metal ions at trace concentration. Different metal ion catalysts like
chromium(III)[4], ruthenium(III)[5], iridium(III)[6] etc.
have been used in the oxidation by cerium(IV). Among the different metal ions,
ruthenium(III) and iridium(III) are highly efficient. In the modern chemical industry, the
higher requirement on catalyst selectivity has been advanced. However, the studies of
active center structure by means of physical method only can not calculate reaction
channel about complicated molecules. Reaction mechanism of various elementary reactions
must be investigated to analyze the effect on selectivity[7]. Therefore, the
basic study of catalytic reaction will prove the scientific basis for improving catalyst
selectivity and making high-efficiency catalyst. Now the lack of any study on the Ce(IV)
oxidation of methanol prompted us to explore the kinetic behavior of the title reaction on
metal ion catalysis in cerium(IV) oxidation reactions.
2 EXPERIMENTAL
Ceric sulfate, ferrous ammonium sulfate, methanol and iridium trichloride were of A.R. grade. Twice distilled water was employed throughout the experiment. Ceric sulfate solution was prepared by warming it in sulfuric acid and standardized with ferrous ammonium sulfate using ferroin as an indicator. Methanol was purified by distillation and its concentration was obtained from its density measurements. Stock solution of iridium trichloride was prepared by dissolving in warm 1.0 mol·dm-3 HCl solution and kept for several days at about 40ºC to attain the equilibrium. The concentration of iridium(III) in the stock solution was determined[8] spectrophotometrically. The ionic strength was maintained by adding KNO3 solution.
2.2 Procedure and kinetic measurements
Under the condition of [CH3OH]>>[Ce(IV)]>>[Ir(III)], 25ml of solution containing definite [Ce(IV)], [Ir(III)], [H2SO4] and [KNO3], and 25ml of methanol solution of appropriate concentration were transferred separately to the upper and lower branch tubes of l type two-cell reactor. After it was thermally equilibrated in a thermostat, the solutions were thoroughly mixed. The progress of the reaction was monitored by withdrawing aliquots of the reaction mixture at regular intervals, quenching the reaction by adding to excess standard ammonium.iron(II) sulfate solution in sulfuric acid and back-titrating the unreacted iron(II) against standard cerium(IV) using ferroin as indicator. The pseudo-first order rate constants kobs were evaluated (Figure 1) from the slope of the plots of ln[Ce(IV)]t versus time (t) i.e. ln((V∞-Vt) versus t where V∞ and Vt devote the volume of standard cerium (IV) solution needed in back titration for the unconsumed iron(II) solution at infinity and time(t) respectively. To evaluate kobs, generally 8-10 values at least up to 80% completion of the reaction were used. Average values of at least two independent determinations of kobs were taken for analysis. The observed rate constants were reproducible within the experimental error 5%.
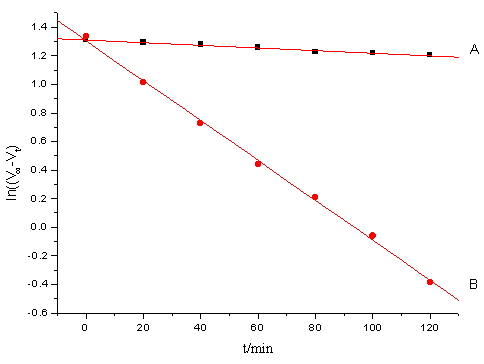
Fig.1 Plots of ln (V∞-Vt) vs. t at 30ºC
[Ce(IV)] = 2.0×10-3mol·dm-3, [CH3OH] = 0.05mol·dm-3, [H2SO4] = 0.5mol·dm-3, m= 1.0mol·dm-3.
A ([Ir(III)] = 0 mol·dm-3); B ([Ir(III)] = 4.0×10-7 mol·dm-3).
3 RESULTS AND DISCUSSIONS
3.1 Dependence on [Ce(IV)]
Under the condition [CH3OH]>>[Ce(IV)]>>[Ir(III)], the plot of ln (V∞-Vt) versus time t was linear(Figure.1), indicating that the reaction is first order with respect to [Ce(IV)]. The pseudo first order rate constants kobs are independent upon the initial concentration of cerium (IV) in the 2 - 4.5 ×10-3mol·dm-3 range. The dependence is given by:
3.2 Dependence on [CH3OH]
At fixed [Ce(IV)], [Ir(III)], [H2SO4], ionic strength (m) and temperature, kobs increases with the increase of [CH3OH]. The plot of kobs vs.[CH3OH] was a smooth curve passing through the origin indicating fractional order in [CH3OH] (observed reaction order nap = 0.2 ). The plot of 1/kobs vs. 1/[CH3OH] exhibits excellent linearity (Figure.2) with a positive intercept and slop.
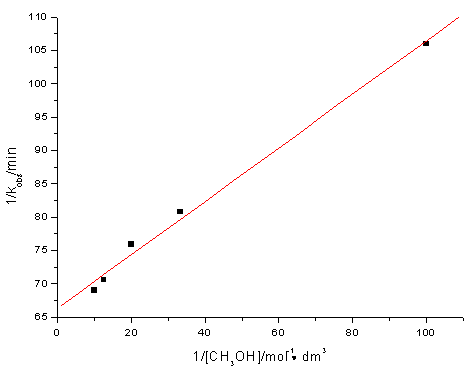
Fig.2 Plot of 1/kobs vs. 1/[CH3OH] at 30ºC
[Ce(IV)] = 2.0×10-3mol·dm-3,[Ir(III)] = 4.0×10-7mol·dm-3,
[H2SO4] = 0.5mol·dm-3, m= 1.0mol·dm-3.
3.3 Dependence on [Ir(III)]
The fact that under the experimental conditions in the absence of Ir(III) ions the reaction practically does not take place is supported by an independent experiment ( Figure.1. A). Addition of traces of Ir(III) enhances the rate significantly (Figure 1. B), kobs shows the fractional order in [Ir(III)] (na p = 0.56-0.69), 1/kobs vs. 1/ [Ir(III)] yielded good linear plots (Figure 3) with a positive intercepts and slops at different temperatures. The kinetic data also fit well with Michaelis-Menten Plot.
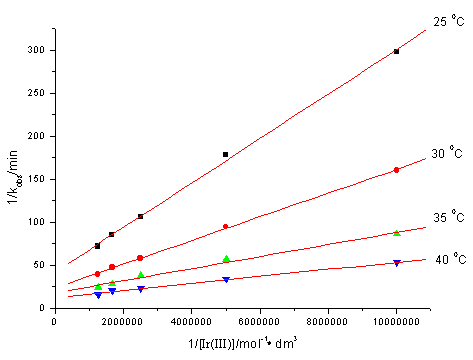
Fig.3 Plots of 1/kobs vs. 1/[Ir(III)] at different temperatures
[Ce(IV)] = 2.0×10-3mol·dm-3, [CH3OH] = 0.05mol·dm-3,
[H2SO4] = 0.5mol·dm-3, m = 1.0mol·dm-3. 3.4 Dependence on [
[
where a, b and c are constants under the experiment conditions.
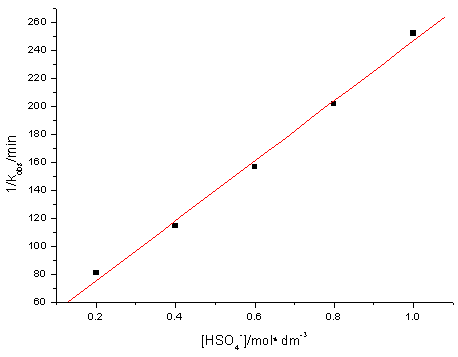
Fig. 4 Plot of 1/kobsvs. [
[Ce(IV)] = 2.0×10-3mol·dm-3, [CH3OH] = 0.05mol·dm-3,
[Ir(III)] = 4.0×10-7mol·dm-3, m= 1.0mol·dm-3.
3.5 Dependence on [H+]
[H+] was varied over the range (0.2-1.0) mol
[H+]/mol·dm-3 0.2 0.4 0.6 0.8 1.0 |
| 103 |
[Ce(IV)]= 2.0×10-3
mol·dm-3, [Ir(III)]= 4.0×10-7 mol·dm-3,
[CH3OH]= 0.05mol·dm-3 , m= 1.0 mol·dm-3,
t = 30ºC
The completion of the reaction was marked by the complete fading of Ce(IV) color (yellow). The reaction product as formaldehyde was detected by spot test[9] and estimated[10] gravimetrically as its 2, 4, dinitrophenylhydrazone derivative. It was found that the reaction is of 1:1 type. Another product Ce(III) was also detected by spot test[11].
3.7 Acrylonitrile polymerization test
Acrylonitrile solution(40%,V/V) was added to the reaction mixture under the protection of nitrogen gas the reaction system can not initiate any polymerization of acrylonitrile indicating no free radical generated in the reaction.
3.8 Mechanism of the reaction
The kinetic data (i.e.1/kobs vs.1/[CH3OH]) fit well with Michaelis-Menten type model suggests that CH3OH is involved in the reversible formation of a complex between the oxidant and the substrate, and the kinetic data (i.e.1/kobs vs.1/ [Ir(III)]) also fit well with Michaelis-Menten type model indicates a probable way of association of Ir(III) and the complex formed in the first preequilibrium step. Ce(SO4)2 has been found kinetically active in this study. Thus a mechanism consistent with the found kinetic characteristics is presented as follows:
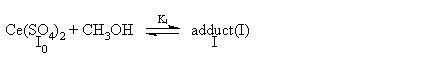 (2)
(2)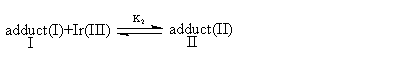 (3)
(3)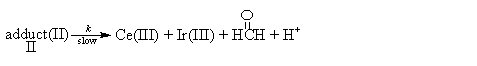 (4)
(4)From the mass balance relationship we have:
[II]e
Subscripts T and e stand for total and equilibrium concentration respectively , f gives the fraction of the total cerium(IV) kinetically active.
From step (4) of the above mechanism the rate of disappearance of [Ce (IV)]T can be written as:
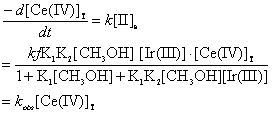 (7)
(7)
![]() (8)
(8)
The other two forms of
Eq.(8) are as follows:
![]() (9)
(9)
![]() (10)
(10)
Eq.(8) can explain well positive fractional order dependence on both
[substrate] and [catalyst]. Eq.(9) suggests that a plot of 1/kobs vs.1/[CH3OH]
at constant [Ir(III)] and [H2SO4] should be linear, and was indeed
evidenced in Figure 2. Eq.(10) shows that the plot of 1/kobs
vs.1/[Ir(III)] at fixed [CH3OH] and [H2SO4] should also
be linear at different temperatures, and the data in Figure 3 proved to be true (r ≥
0.996)
kn (kn=fk), K1 and K2
from the intercepts and slops of lines in Figure 3 and Figure 2 were evaluated. kn
at different temperatures and activation parameters are listed in Table 2.
Table 2 Rate constants and activation parameters
t / ºC |
25 |
30 |
35 |
40 |
activation parameters (25 ºC) |
102 kn/ min-1 |
2.38 |
4.27 |
5.63 |
8.19 |
Ea*=62KJ ·mol-1, DH≠=59KJ·mol-1DS≠= -76J·K-1·>mol-1,DG≠=86KJ·mol-1 |
* r = 0.99, B = -7449, A = 21.32 for the linear regression of ln kn vs.1/T
The second equilibrium probably involves the outersphere association of the species(I) and catalyst followed by the electron transfer leading to the species(II) which may be Ce(III)·(CH3OH)·Ir(IV), subsequently electron transfer occurs within the complex to give Ir(III) and the free radical which is rapidly oxidized by Ce(IV) at a fast step. Thus the oxidation of methanol occurs through the Ir(III)/Ir(IV) catalytic cycle. 3.9 Kinetically active Ce(IV) species and [Under the experimental conditions in aqueous sulfuric acid medium, the important cerium(IV)-sulfato complexes are Ce(SO4)2+ , Ce(SO4)2 and HCe(SO4)
Ce4++
Ce(SO4)2 +
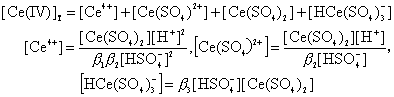 (14)
(14)Substituting above Eqs. into Eq.(14) we get:
By considering the relative magnitudes of the successive formation equilibrium constants which are in the order
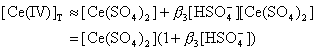 (16)
(16)
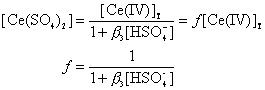 (17)
Substituting Eq.(17)into Eq.(8)we get:
(17)
Substituting Eq.(17)into Eq.(8)we get:
where
Eq.(19) can be derived from Eq.(18) after rearrangement:
or
Eq.(19) is the same as Eq.(1) and can explain well negative number order (na p= - 0.70) dependence on [
Because of the existence of so many protondependent equilibria[12] among the reactants, the quantitative interpretation of [H+] dependence is very much complicated and uncertain. Because of this complexity in the present system, no attempt was made to explain the observed [H+] dependence from the proposed reaction mechanism. However, the qualitative observation is in agreement with the fact that cerium(IV) oxidation reaction in aqueous sulfuric acid media are acid catalyzed. On protonation, positive charge on the cerium(IV) sulfato species increases and it facilitates the electron transfer towards cerium(IV) center.
REFERENCES
[1] Song W Y, Li Z H, Wang A Z. Chemical Journal of Chinese Universities ( Gaodeng Xuexiao
Huaxue Xuebao), 1997, 18 (11) : 1842.
[2] Song W Y, Bai S Y, Zhang L M. Chinese Journal of Inorganic Chemistry ( Wuji Huaxue
Xuebao), 2002, 18 (5) : 451.
[3] Lakshmi S, Renganathan R. International Journal of Chemical Kinetics.1996, 29 : 713.
[4] Song W Y, Jiang Q M. Acta Chimica Sinica, (Huaxue Xuebao), 2005, 63(2) : 109.
[5] Das A K, Das M. International Journal of Chemical Kinetics, 1995, 27 : 7.
[6] Song W Y, Li H B, Liu H M. Acta Physico-Chimica Sinica (Wuli Huaxue Xuebao). 2004, 20
(8) : 801.
[7] Guo X X. Chemical Bulletin ( Huaxue Tongbao), 1979, 6 : 15.
[8] Singh A K, Katyal R P, Mohan R P. Journal of Indian Chemical Society, 1976 : 691.
[9] Feigl F. Spot Tests in Organic Analysis, New York : Elsevier Publishing Co., 1956.
[10] Vogel V I. in Quantitative Organic Analysis (Part 3), London : ELBS, 1958.
[11] Department of Chemistry, Hangzhou University. Handbook of Analytical Chemistry,
Beijing : Chemical Industry Press, 1982.
[12] Misra S K, Gupta Y K. Journal of Chemical Society A, 1970.
[13] Bugaenko L T, Kuan-Lin H. Russian Journal of Inorganic Chemistry, 1963, 8 : 1299.
铱(III)离子催化铈(IV)离子氧化甲醇的反应动力学及机理
宋文玉 赵荣慧 李彦维 徐俊然
(河北大学化学与环境科学学院,保定
071002)
摘要 在酸性介质中用氧化还原滴定法研究了铈 (IV)
离子在痕量铱 (III) 离子催化作用下,于25-40℃区间氧化甲醇的反应动力学. 结果表明反应对铈
(IV) 离子为一级,对甲醇和铱 (III) 离子的表观反应级数均为正分数.
准一级速率常数([CH3OH]>>[Ce(IV)])kobs随 [H+]
增加而增大,而随 [![]() ] 增加而减小.
在氮气保护下,反应不能引发丙烯腈聚合,说明在反应中没有自由基产生.通过kobs与
[
] 增加而减小.
在氮气保护下,反应不能引发丙烯腈聚合,说明在反应中没有自由基产生.通过kobs与
[![]() ]
的依赖关系,找到本反应体系的动力学活性物种是Ce(SO4)2,并计算出平衡常数,速控步骤的速率常数及相应的活化参数.
]
的依赖关系,找到本反应体系的动力学活性物种是Ce(SO4)2,并计算出平衡常数,速控步骤的速率常数及相应的活化参数.
关键词 铱 (III) 离子, 铈 (IV) 离子, 甲醇, 催化剂,
动力学及机理