http://www.chemistrymag.org/cji/2005/07c082pe.htm |
Dec. 2, 2005 Vol.7 No.12 P.82 Copyright |
Wu Xianhua, Zhong Yihui, Zhang Xin, Huang Qiling
(Department of Chemistry, Yuxi Teacher's College, Yunnan 653100, China)
Received on Sep. 25, 2005.
Abstract In this paper, a new chromogenic reagent, 5-(p-aminobenzylidene)-thiorhodanine (ABTR) was synthesized. A high sensitive, selective and rapid method for the determination of gold based on the rapid reaction of gold with ABTR and the solid phase extraction of the colored chelate with a reversed phase polymer-based C18 cartridge has been developed. In the presence of 0.05-0.5 mol L-1 of hydrochloric acid solution and emulsifier-OP medium, ABTR reacts with gold to form a red chelate of a molar ratio 1:3 (gold to ABTR). This chelate was enriched by the solid phase extraction with a polymer-based C18 cartridge and eluted to form the chelate from cartridge with dimethyl formamide (DMF). The ABTR-Au(III)) chelate in 100 mL solution can be concentrated to 1.0 mL, and the enrichment factor of 100 was achieved. In the DMF medium, the molar absorptivity of the chelate is 1.23×105 L.mol-1.cm-1
at 550 nm. Beer's law is obeyed in the range of 0.01- 3 mg mL-1 in the measured solution. The relative standard deviation for eleven replicate samples of 0.5 mg L-1 level is 2.18%. The detection limit, based on the three times of standard deviation is 0.02 mg L-1 in the original sample. This method was applied to the determination of gold in water and ore with good results.Keywords Gold, solid phase extraction; spectrophotometry, 5-(p-aminobenzylidene)-thiorhodanine
1. INTRODUCTION
Gold belongs to the elements which occur on the Earth with very low natural contents. It
is one of most important noble metals due to its wide application in industry and economic
activity. For this reason, a simple, sensitive and selective method for determination of
trace gold was required strongly. Although several sophisticated techniques, such as
inductively coupled plasma mass spectrometry (ICP-MS), inductively coupled plasma atomic
emission spectrometry (ICP-AES), electrochemical, spectrofluorimetry, neutron activation
analysis and the like have widely been applied to the determination of gold
Solid phase extraction is an attractive technique because of its notable advantages. In our previous works, the determination of some trace metal ions by solid phase extraction with reserved phase silica-bond with C18 cartridge was studied [19-23]. However, the routine reserved phase silica-bond C18 cartridge only can be used in pH range of 2 - 8. The chromogenic systems in acidic or alkaline medium can not be preconcentrated by this cartridge. To meet the need of metal chelate enrichment by solid phase extraction in acid medium, in this work, the color reaction of 5-(p-aminobenzylidene)-rhodanine (ABTR) with gold and the solid phase extraction of Au-ABTR chelate with a reversed phase polymer-based C18 cartridge was studied. The polymer-based C18 is manufactured from a hydrophilic methacrylate polymer, which is functionalized with C18 ligands. It is a reversed-phase solid phase cartridge provides a broad range of solvent choices and a pH range from 0 - 14. By using the polymer-based C18 cartridge, the Au-ABTR chelate was enriched by solid phase extraction in hydrochloric acid medium and the enrichment fact of 100 was achieved. Based on this, a highly sensitive, selective and rapid method for the determination of gold in water and ore samples was developed.
2. EXPERIMENTAL
2.1 Apparatus
A UV-160 A spectrophotometer (Shimidzu Corporation,
Tokyo, Japan) equipped with 1 cm microcells (0.5 mL) was used for all absorbance
measurements. The pH values were determined with a Beckman F-200 pH meter (Beckman Instruments, Fullerton, CA, USA). The
extraction was performed on Waters Solid Phase Extraction (SPE) device (It can prepare
twenty samples simultaneously), and a reversed phase Polymer C
2.2 Reagents
The ABTR was synthesized according to our previously proposed procedure [24]: 40 mL of acetic acid was added to the sample of 1.5 g of thiorhodanine and 1.2 g of p-aminobenzaldehyde, and the mixture was heated gently to dissolve the thiorhodanine and p-aminobenzaldehyde completely. The solution was refluxed for about 1.5 h. In the course of refluxing, 1 mL of concentrated sulfuric acid was added dropwise. After the color of the solution turned red, the refluxing was stopped and the sample was poured into 200 mL of distilled water. To this solution, a small amount of aqueous ammonia was added. Thereafter, the precipitants were separated by filtration, and were recrystallized twice with absolute alcohol. The yield is 55% (m. p. 282oC-285oC). The structure of ABTR was verified as shown in Fig.1 by elemental analysis, IR, 1HNMR and MS. Elemental analysis: calculated (found), 47.59 (47.23)% C, 3.19 (3.28)% H, 11.10 (11.02)% N, 38.12 (37.53)%. IR (KBr) (cm-1): 3470, 3450, 3355 (nN-H); 3060, 3020 (n-c=C-H); 1628 (dN-H); 1566, 1548, 1515, 1450 (nC=C); 1292 (nC-N); 1171 (nC=S); 825 (dAr-H); 806 (dC=C-H). 1HNMR (solvent: DMSO-d6) (d,ppm): 7.46 (1H, s, C=C-H); 7.26, 7.35 (2H, d, J≈9Hz, H-2 and H-6); 6.62, 6.72 (2H, d, J≈9Hz, H-3 and H-5); 3.36 (2H, w, -NH2). MS (EI) (m/z): 252 (M+·).
All solutions were prepared with ultra-pure water obtained from a Milli-Q50 SP Reagent Water System (Millipore Corporation, USA). High purity dimethyl formamide (DMF) (Fisher Corporation, USA) was used. A 3.0×10-4 mol L-1 of ABTR solution was prepared by dissolving ABTR in DMF. A stock standard solution of gold (1.0 mg mL-1) was obtained from Chinese Material Standard Center, and a work solution of 0.5 m g mL-1 was prepared by diluting this solution. A solution of 5 mol L-1 of hydrochloric acid was used. Emulsifier-OP solution (2.0 % (v/v)) was prepared by dissolving emulsifier-OP with water. All chemical used were of analytical grade unless otherwise stated.
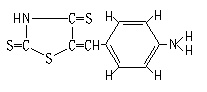
Fig.1 The structure of ABTR
2.3 General Procedure
To a standard or sample solution containing no more than 3.0 m g of Au(III) in a 100 mL of
calibrated flask, 5 mL of 5 mol L-1 of hydrochloric acid solution, 5.0 mL of
3.0×10-4 mol L-1 ABTR solution and 3.0 mL of 2.0 % emulsifier-OP
solution were added. The mixture was diluted to volume of 100 mL and mixed well. After 10
min, the solution was passed through the polymer-based C18 cartridge at a flow
rate of 20 mL min-1. After the enrichment was finished, the retained chelate is
eluted from the cartridge at a flow rate of 5 mL min-1 with 1.0 mL of DMF in
the reverse direction. The eluent was adjusted to the accurate volume of 1.0 mL in a 1.0
mL calibrated flask by adding microamount of DMF with a 200 m l syringes. The absorbance
of this solution was measured at 550 nm in a 1cm microcells (0.5 mL) against a reagent
blank prepared in a similar way without gold.
3. RESULTS AND DISCUSSION
3.1 Absorption Spectra
The absorption spectra of ABTR and its Au(III) chelate are shown in Fig.2. The absorption
peaks of ABTR and its complex in DMF medium are located at 430 nm and 550 nm.
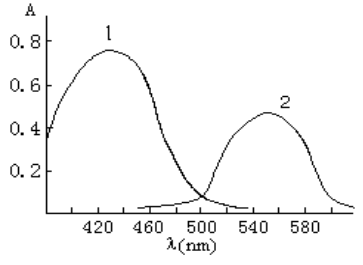
Fig.2 Absorption spectra of
ABTR and its Au(III) complex: 1 ABR-emulsifier-OP blank against water; 2
ABR-emulsifier-OP-Au(III) chelate against reagent blank.
3.2 Effect of Acidity
Results showed that the optimal condition for the reaction of Au(III) with ABTR is in the
acid medium. Therefore, the effect of hydrochloric acid, sulfuric acid, perchioric acid,
phosphoric acid and the like, on the color reaction of Au(III) with ABTR was studied.
Experiment shows that hydrochloric acid has the best effect, and the concentration of
hydrochloric acid within a 0.05-0.5 mol L-1 was found to give a maximum and
constant absorbance, so 5 mL of hydrochloric acid was recommended.
3.3 Effect of Surfactants
The effects of surfactants on Au(III)-ABTR system were studied. The results (Table 1)
showed that in the absence of surfactants, anionic surfactants or cationic surfactants,
the Au(III)-ABTR chromogenic system gives a low absorption, whereas in the presence of
nonionic surfactants, the absorption of the chromogenic system increases markedly. Various
anionic surfactants enhance the absorbance in the following sequence: Emulsifier-OP >
Tween-80 > Tween-20 > Tween-60. Accordingly, the emulsifier-OP was the best
additive, and the use of 1.5 - 4 mL of emulsifier-OP solution give a constant and maximum
absorbance. Consequently, the use of 3.0 mL was recommended.
Surfactant |
Absence |
Emulsifier-OP |
Tween-80 |
Tween-20 |
Tween-60 |
SDS |
CTMAB |
CPB |
l max (nm) |
530 |
550 |
545 |
540 |
535 |
530 |
535 |
525 |
| e (×104) L.mol-1.cm-1 |
8.26 |
12.3 |
9.18 |
10.2 |
8.36 |
7.18 |
5.84 |
6.85 |
3.4 Effect of ABTR
Concentration
For up to 3.0 m g of Au(III), the use of 5 mL of 3.0×10-4 mol L-1
of ABTR solution was found to be sufficient for a complete reaction. Accordingly, 5.0 mL
of ABTR solution were added in all further measurement.
3.5 Stability of the Chromogenic System
After mixing the components of the system, the absorbance reaches its maximum within 8 min
at room temperature and remains stable for at least 5 h. After having been extracted into
the DMF medium, the chelate was stable for at least 8 h.
3.6 Solid Phase Extraction
Both the enrichment and the elution were carried out on a Waters SPE device (which can
prepare twenty samples simultaneously). The flow rate was set to 20 mL min-1
when enrichment and 5 mL min-1 when elution.
Some experiments were carried out in order to investigate the retention of
ABTR and its Au(III) chelate on the cartridge. It was found that the ABTR and its Au(III)
chelate was retained on the cartridge quantitatively when they pass the cartridge as
hydrochloric acid medium. The capacity of the cartridge was determined as 22 mg for
Au(III)-ABTR chelate in a 100 mL of solution. In this experiment, the maximum amount gold
is only 3.0 m g. Therefore, the cartridge has adequate capacity to enrich the Au(III)-ABTR
chelate.
In order to choose a proper eluant for the retained ABTR and its Au(III) chelate. Various organic solvents were studied. For eluting the Au(III)-ABTR chelates from the cartridge, the volume of the solvent needed is 0.8 mL for DMF, 1.2 mL for isopentyl alcohol, 1.4 mL for acetone, 1.4 mL for acetonitrile, 1.8 mL for ethanol, 2.0 mL for methanol. The maximal enrichment was achieved when DMF was selected as eluant. So the DMF was selected as eluant. The experiment show that it was easier to elute the retained ABTR and its Au(III) chelate in reverse direction than in forward direction(Only 0.8 mL of eluant was needed when eluted in reverse direction. However, 2.2 mL of eluant was needed when eluted in forward direction), so it is necessary to upturn cartridge when elution. 1.0 mL of DMF was sufficient to elute the ABTR and its Au(III) chelate from cartridge at a flow rate of 5 mL min-1. The volume of 1.0 mL was selected.
3.7 Calibration Curve and Sensitivity
The calibration curve showed that Beer's law is obeyed in the concentration range of 0.01- 3 m g Au(III) per mL in the measured solution. The linear regression equation obtained was A = 0.621 C (m g mL-1) + 0.0154 (r =0.9992). The molar absorptivity was calculated to be 1.23×105 L mol-1 cm-1 at 550 nm. The relative standard deviation at a concentration level of 0.5 m g L-1 of Au(III) (11 repeats determination) was 2.18%. The detection limit, based on the three times of standard deviation, is 0.02 m g L-1 in the original sample.
3.8 Interference
The selectivity of the proposed method was investigated by the determination 0.5 m g 100 mL-1 of Au(III) in the presence of various ions within a relative error of ± 5% are given in Table 2. The result shows that most common ions do not interfere with the determination. This method is highly selective. Table 2 Tolerance Limits for the Determination of 1.0 m g of Au(III) with ABTR
(relative error ± 5%)
Ion added |
Tolerate (mg) |
NO3-, K+, borate, Na+ |
50 |
Li+, Al3+, PO43-, NO2-, SO42-, ClO4- |
20 |
Ca2+, Mg2+, SO32-, Sr2+, Ba2+, IO3-, BrO3-, ClO3- |
10 |
Mn2+, Ce(IV), W(VI), Mo(VI), U(IV), Fe3+ |
4 |
Ti(IV), Bi(III), V(V), Cr(VI), Zr(IV), F-, Fe2+, Cl- |
1 |
Cd2+, Cr3+, La3+, Sn(IV), Zn2+, Zr(IV) , Co2+, Ni2+ |
0.5 |
Ru(III), Bi(III), Pb2+, Sb3+, Th(IV), Br-, Os(VIII) , I-, Cu2+ |
0.2 |
Se(IV), Te(IV), S2O32-, Ag+ |
0.1 |
Ir(IV) , Rh(III), Ru(III) |
0.05 |
Pt(IV), Hg2+ |
0.01 |
CN-, SCN- |
0.005 |
Table 3 Determination of gold in the water and ore samples
Samples |
ICP-MS method |
Found |
RSD% (n=5) |
Recovery (%) (n=5) |
River water |
4.86 (mg L-1) |
4.67(mg L-1) |
3.6 |
89 |
Planting effluents |
31.6 (mg L-1) |
32.9 (mg L-1) |
2.8 |
94 |
Ore |
0.358 (g T-1) |
0.389 (g T-1) |
3.4 |
92 |
Ore |
3.62 (g T-1) |
3.56 (g T-1) |
3.1 |
95 |
3.9 Composition of the Complex
The composition of the complex was determined by continuous variation and molar ratio
method. Both showed that the molar ratio of Au(III) to ABTR is 1:3.
3.10 Application
Applied to the water samples
For water sample, the samples were acidified with hydrochloric acid and filtrated by
0.45 m m filter. The gold contents were analyzed according to general procedure. The
results (deducted the reagents blank) are shown in Table 2. An ICP-MS method was used as a
reference method and the results are shown in Table 2, too.
Applied to the ore samples
A 1.0 g of ore sample is weighed into a 50 mL of teflon high-pressure microwave
acid-digestion bomb (Fei Yue Analytical Instrument Factory, Shanghai, China). To which, 10
mL of aqua regia was added. The bombs were sealed tightly and then positioned in the
carousel of the microwave oven (Model WL 5001, 1000 W, Fei Yue Analytical Instrument
Factory, Shanghai, China). The system was operated at full power for 30 min. The digested
material was evaporated to incipient dryness. Then, 20 mL of 5% hydrochloric acid was
added and heated close to boiling to leach the residue. After cooled, the residue was
filtrated and the undissolved residure was washed with 5% hydrochloric acid for two times.
The leachate was collected into a 100 mL calibrated flask quantitatively and the gold
contents were analyzed according to general procedure. The results (deducted the reagents
blank) were also shown in Table 2. An ICP-MS method was used as a reference method and the
results are also shown in Table 2.
4. CONCLUSION
This method has the merits of high selectivity and high sensitivity. ABTR is a
sensitive and selective spectrophotometric reagent for gold. The molar absorptivity of the
chelate reaches 1.23×105 L mol-1 cm-1. Most foreign ions
do not interfere with the determination. By solid phase extraction with C18
cartridge, the ABTR-Au(III)) chelate in 100 mL solution can be concentrated to 1.0 mL, the
enrichment fact of 100 was achieved. The detection limit reaches 0.02 m g L-1
in the original samples, and m g L-1 level of gold content can be determined
with good results.
REFERENCES
[1] Shigeru T S, Masahiro T C. Bunseki Kagaku,1999, 48: 847-854.
[2] Malhotra RK, Ramanaiah G V, Satyanarayana K. At Spectrosc,1999, 20: 92-102.
[3] Zuzana N, Petr K. Fresenius J. Anal Chem., 2000, 367: 369-372.
[4] Lack B, Nyokong T, Duncan J. Anal Chim Acta,1999, 385: 393-399.
[5] Ye RD, Khoo S B. () Analyst, 1999, 124:353-360.
[6] Wang CM, Sun Y, Li HL, Zhang HL. Anal Chim Acta,1998, 361: 133-139.
[7] Dequaire M, Degrand C, Limoges B. Anal Chem, 2000, 72: 5521-5528.
[8] Kiwamu O, Gen S, Yoshiyuki T. Chem Geol., 1999, 157: 189-197.
[9] Gunther D H, Christoph A. J Anal At Spectrom, 1999, 14: 1363-1368.
[10] Anjali P. Talanta,1998, 46: 583-587.
[11] Koh T, Okazaki T, Ichikawa M. Anal Sci., 1986, 2: 249-254.
[12] Zeng Z T, Xu Q H. Talanta, 1992, 39: 409-413.
[13] Lichtenstein I E. Anal Chem, 1975, 47: 465.
[14] EI-Zawawy F M, EI-Shahat M F, Mohamed A A et al. Analyst, 1995, 120: 549.
[15] Long G L, Wineforder J D. Anal Chem,1983, 55: 712A.
[16] Long G L, Wineforder J D. Anal Chem, 1980, 52: 2242.
[17] Medinilla J, Ales F, Sanchez FG. Talanta, 1986, 33: 329.
[18] Li Z J, Pan J M, Tang J. Anal Bioanal Chem, 2003, 375: 408-413.
[19] Hu Q F, Yang G Y, Yin J Y. J. Environ Monit, 2002, 4: 956-959.
[20] Hu Q F, Yang G Y, Yin J Y. Talanta, 2002, 57: 751-756.
[21] Hu Q F, Yang G Y, Huang Z J, Yin J Y. Talanta, 2002, 58: 467-473.
[22] Yang G Y, Dong X C, Hu Q F, Yin J Y. Anal Lett, 2002, 35: 1735-1745.
[23] Li Z, Yang G Y, Wang B X, Yin J Y. J Chromatogr A, 2002, 971, 243-247.
[24] Hu Q F, Wu X H, Huang Z J et al. S Afr J Chem.,2005, 58, 64-69.
吴献花,仲一卉,章 新,黄齐林
(玉溪师范学院化学与环境科学系,玉溪 653100)
摘要 本文合成了新试剂对氨基苯亚甲基硫代若丹宁 (ABTR),并研究了ABTR与金的显色反应,在0.05-0.5 mol/L的盐酸介质中,乳化剂-OP存在下,ABTR与金反应生成2:1稳定络合物,该络合物可用polymer-based C18聚合物键合固相萃取柱富集,用DMF洗脱后可用光度法测定,在DMF介质中,该络合物的lmax=550 nm,e=1.23×10 5 L.mol-1.cm-1,金的含量在0.01~3 m g/mL内符合比尔定律,方法的检测限达0.02 mg/L,方法用于环境水样和矿石中金的测定,结果令人满意。
关键词 对氨基苯亚甲基硫代若丹宁;金;光度法;固相萃取