http://www.chemistrymag.org/cji/2006/081005pe.htm |
Jan. 10, 2006 Vol.8 No.1 P.5 Copyright |
(Department of Chem. & Chem. Eng., Baoji University of Arts and Science, Baoji, Shaanxi 721007, China)
Abstract By means of solvent
partition, preparative TLC and spectral analysis, a main active substance with the
biological activity was fraxinellone contained in D.dasycarpus. Its antifeeding
rate and mortality of 72h at 0.21% (mass percent) against 3rd armyworm (mythimna
separata) were 90.4% and 95.2%, respectively. Then antimicrobial activity of
fraxinellone had been tested against 7 kinds of bacteria and 10 kinds of plant diseases.
The inhibition rate of fraxinellone at 50ppm reached 100%, 99.8% and 99.8% against
Escherichia coli, Staphlococcus aurens and Bacillus megathrium, respectively. Its
inhibition rate at 100ppm against Rhizotonia cerealis van der Hoeven apud.Boerema &
Verhoeven was 100% and at 50ppm against Colletrichurm lagenarium & verhoeven was 72%.
Keywords Dictamnus dasycarpus Turks, Fraxinellone, Pesticidal Activity,
Antimicrobial Activity, Mythimna separata, Bacteria, Plant Diseases.
1. INTRODUCTION
Dictammus dasycarpus Turks belonging to the family Rutaceae has the function of
detoxification, anticancer and antibacterial and is used for jaundice and skin diseases
[1]. The mutagenic and pharmacological activities of the total alkaloids, dictamnine,
skimmianine and g-fagarine
from Dictamni Radicis Cortex have been reviewed [2,3]. Fraxinellone and dictamnine
were shown to have pharmacological activities, antiplatelet aggregation as well as being
effective vasorelaxants [4-7]. The isolation and biological properties of fraxinellone
from Rutaceae and Meliaceae have been reported [8-14]. Liu, et al [15] studied feeding
deterrents of fraxinellone against two stored-product insects (T. castaneum and
S.zeamais) using a flour disk bioassay.
This paper investigated the pesticidal activity of fraxinellone from D.
dasycarpus against 3rd armyworm (Mythimna separata) using the leaf disk method
[16]. At the same time, its fungicidal activity against 7 kinds of bacteria and 10 kinds
of plant diseases had been studied.
2.1 Materials
D. dasycarpus was herborized from Taibai Mountain located in Qingling Mountain, Shaanxi, P.R.China, in July 2003. The plant material was identified by Dr. Yin-ping Zhao, Department of Biology, Xian United University. The plant specimens, which have been dried in air and powdered, are deposited in the Herbarium of the Key Laboratory of Phytochemistry, Baoji University of Arts and Science, Baoji, Shaanxi (No. DRC-0306). All the reagents used were of analytically pure grade.
2.2 Apparatus
HR-FABMS and EIMS mass spectra were recorded in an Autospec 3000 instrument and HP5988A mass, respectively. 1H and 13C NMR spectra were measured on BRUKER AM-400 Nuclear Magnetic Resonance Spectrometer. The chemical shift values are given in ppm using TMS as the internal standard. Melting points were determined on a MP-J3 micro melting point apparatus.
2.3 Ascertain and Preparation of active substance
The powder of D. dasycarpus (1.2kg) was wetted with 10% lime milk and extracted 3 times with methanol. The extractive had been filtrated and concentrated to afford a residue (48.9g). By solvent partition and bioassay, the fast rate and mortality rate of the extract petroleum ether were topmost and come up to 100% and 79.3%, respectively. So the residue (12.7g) of petroleum ether extractive was partitioned on a basic silica gel (Merck 9385, 1000 g) column using methanol-petroleum ether (3+1 by volume) as eluent provided 4 fractions. These substances were purified further by preparative TLC (10cm×15cm) with cyclohexane + ethyl acetate (3.5+2) as eluent. A colourless raphide S1 (1.4027g) was recrystallized 3 times from methanol (20ml). The colourless raphide was identified and elucidated as fraxinellone by HPLC and its spectral data. Its pesticidal activity and bactericidal activity were tested by the leaf disk method and the paper scrap method.
2.4 Testing Pesticidal Activity ---- the leaf disk method [16]
A fresh corn blade about 1 cm2 was put in the sample and awaited for 1-2s, then took it out and dried in air in reserve. Every kind of the sample was tested on ten Mythimna separata, meanwhile contrasted with corresponding solvent and repeated three times. The ten Mythimna separata on eating and death situation after 24h, 48h, 72h were checked in the Breeding Room (T = 25 ±1ºC, RH = 70-80ºC, L/D = 12h/12h). Then antifeeding rate and mortality rate were calculated by the following formula.
Antifeeding rate(%)={[Check trophic exponent(%)-Disposal trophic exponent(%)]/Check trophic exponent(%)} ×100﹪
Mortality(%)= [ Death worm number /Test worm gross ]×100﹪
2.5 Testing Bactericidal Activity ---- the paper scrap method
Using the paper scrap method [17], PSAY (potato, saccharose, agar and yeast) as culture medium, the bacillus for testing are seven kinds (Escherichia coli; Staphlococcus aurens; Bacillus subtilis; Serratia marcescens; Bacillus megathrium; Bacillus mycoides; Proteus vulgaris), and the fungi for testing are ten kinds [Exserohilum turcicum; Gibberella zeae; Verticillium albo-atrum; Alernaria brassicae; Colletrichurm lagenarium & verhoeven; Alternaria alternata Phytophora infestans(Mont.)De Bary; Rhizotonia cerealis van der Hoeven apud.Boerema & Verhoeven; Bipolaris sativum; C.diplodiel; Alternaria solani(Ellis et Martin)Jones et Grout]. 3. RESULTS AND DISCUSSION
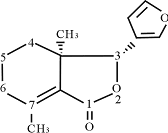
Table 1 1H-NMR and 13C-NMR data for compound S
1 (CDCl3, 400 MHz)Position |
1H | 13C |
1 |
170.15 (s) |
|
3 |
4.88 (1H, d, br ) |
83.65 (d) |
4 |
1.60, 1H, m (4a); |
43.21 (t) |
5 |
1.694 -1.803, 2H, m |
20.55 (t) |
6 |
2.26, 1H, dd, J=19.6, 6.4 (6a); 2.21, 1H, m (6b) |
31.86 (t) |
7 |
148.83 (s) |
|
8 |
127.58 (s) |
|
9 |
32.30 (s) |
|
2' |
7.44 (d, br) |
140.00 (d) |
3' |
120.80 (s) |
|
4' |
6.34 (d, J=0.7 ) |
108.77(d) |
5' |
7.47 (d, J=0.7 ) |
143.65 (d) |
7-CH3 |
2.15 (3H, s ) |
18.44 (q) |
9-CH3 |
0.85(3H, s ) |
18.69 (q) |
3.1 Structure Determination of
Fraxinellone
Compound S1 was colorless needles, mp 113-114℃ and
exhibited [M+1]+ at m/z 233.1172 corresponding to a molecular formula C14H16O3
by positive HR-FABMS and 13C-NMR spectroscopic data (DEPT). Its 1H-NMR
and 13C-NMR spectrum is corresponding to a known compound named fraxinellone
[11-13]. So the compound S
fraxinellone (compound S1)
Colorless needles, m.p.: 113-114℃. HR- FABMS: m/z 233.1172 [M+1]+ for C14H16O3 (calc. 233.2865). EIMS m/z (rel. int.): 232 [M]+ (2), 233[M+1]+ (0.3), 234[M+2]+ (0.03), 136(75), 108(39), 93(100), 91(15). 1H and 13C NMR spectra data are listed in Table 1.
Table 2 The pesticidal activity of fraxinellone at different concentrations
Sample concentration |
Total Amount of 3rd Armyworm (Head) |
Antifeeding Rate(%) |
72h |
||
24h 48h 72h |
|||||
0.08 |
29 |
32.2 |
46.3 |
55.0 |
0.0 |
0.15 |
29 |
60.1 |
53.0 |
54.1 |
85.2 |
0.21 |
30 |
62.8 |
71.4 |
90.4 |
95.2 |
0.49 |
30 |
88.3 |
100.0 |
100.0 |
100.0 |
Table 3 The antimicrobial effects of fraxinellone against seven kinds of bacillus (the inhibition rate: %)
Dilutability (g/ml) |
The bacillus for testing |
||||||
Ec |
Sa |
Bs |
Sm |
Bme |
Bmy |
Pv |
|
0.0500/500 |
- |
- |
- |
- |
- |
- |
- |
0.0500/750 |
63.4 |
59.6 |
- |
57.4 |
- |
- |
|
0.0500/1000 |
100 |
99.8 |
- |
- |
99.8 |
- |
- |
0.0500/1500 |
- |
- |
- |
- |
- |
- |
- |
Axenic water |
- |
- |
- |
- |
- |
- |
- |
Table 4 The antimicrobial effects of fraxinellone against ten kinds of fungi (the inhibition rate: %)
Dilutability (g/ml) |
The bacillus for testing |
|||||||||
Et |
Gz |
Va |
Ab |
Cl |
Aa |
Rc |
Bs |
Cd |
As |
|
0.0500/500 |
- |
- |
- |
- |
- |
- |
100 |
- |
- |
- |
0.0500/750 |
- |
- |
- |
- |
56.0 |
- |
84.3 |
- |
- |
- |
0.0500/1000 |
- |
- |
- |
- |
72.0 |
- |
77.0 |
- |
- |
- |
0.0500/1500 |
- |
- |
- |
- |
33.3 |
- |
44.5 |
- |
- |
- |
Axenic water |
- |
- |
- |
- |
- |
- |
- |
- |
- |
- |
3.2 Bioactivities of fraxinellone
The pesticidal activity of fraxinellone against Mythimna separata was also
demonstrated by the bioassays using the leaf disk method. As shown in Table 2,
fraxinellone at different concentrations exhibited bioactivity and the optimum
concentration was at 0.21%.
The results of bactericidal activity of fraxinellone tested against
seven kinds of bacillus and ten kinds of fungi were shown in Table 3 and Table 4.
Fraxinellone at 100ppm had no effect against seven kinds of bacillus but the inhibition
rate of fraxinellone at 50ppm reached 100%, 99.8% and 99.8% against Escherichia coli,
Staphlococcus aurens and Bacillus megathrium, respectively.
It was shown in Table 4 that Fraxinellone against most of ten kinds of
fungi for testing showed no antimicrobial effect. Its inhibition rate against Rhizotonia
cerealis van der Hoeven apud.Boerema & Verhoeven at 100ppm was 100% and at 50ppm
against Colletrichurm lagenarium & verhoeven was 72%.
Above data indicated that fraxinellone has better biological actions.
In the previous papers, fraxinellone has been known for a long time as a degraded
limonoids[18,19]. They have attracted considerable attention because of their various and
significant biological properties [10-22] and are considered as prototypes for
insecticide. Our research confirmed that fraxinellone was the active substance having
pesticidal activity and bactericidal activity resulting from D.dasycarpus.
REFERENCES
[1] Baoji Public Health Bureau. Annals of
Chinese Taibai Mountain Medical Material. Xian: Shaanxi Scientific Technology Publishing
House, 1992.
[2] Mari Mizuta and Hisayuki Kanamori. Mutation Research, 1985, 144: 221-225.
[3] Nieschulz and Georg Schneider. Naturwissenschaften, 1965, 52: 394-395.
[4] Otto Nieschulz. Sci. Pharm. Proc, 1966, 2: 559-564.
[5] Yu S M, Ko F N, Su M J et al. Pharmacology, 1992, 345: 349-355.
[6] Blaise A J, Winternitz F. Phytochemistry, 1985, 24: 2379-2381.
[7] Boustie J, Moulis C, Gleye J et al. Phytochemistry, 1990, 29: 1699-1701.
[8] Woo W S, Lee E B and Kang S S. Planta Med., 1987, 399-401.
[9] Nakatani M, Huang R C, Okamura H et al. Phytochemistry, 1998,49: 1773-1776.
[10] Okamura H, Yamauchi K, Miyawaki K et al. Tetrahedron Letters, 1997, 38: 263-266.
[11] Coggon P, Mcphail A T, Storer R et al. Chem. Commun. 1969, 828.
[12] D'Ambrosio M, Guerriero A. Phytochemistry, 2002, 60: 419-424.
[13] Blaise A J, Winternitz F. Phytochemistry, 1985, 24: 2379-2381.
[14] Wu T S, Li CY, Leu Y L et al. Phytochemistry, 1999, 50: 509-512.
[15] Liu Z L, Xu YJ, Wu J et al. Journal of Agricultural and Food Chemistry, 2002, 50:
1447-1450.
[16] Wu W J, Liu HX, Zhu J B et al. Natural products insecticides --Theory, method and
practice. Xian: Shaanxi Scientific Technology Publishing House, 1998.
[17] Huang J P, Lin Q X and Song X N. Journal of Central South Forestry University, 1999,
19: 82-84.
[18] Dreyer D L, Pickering M V and Cohan P. Phytochemistry, 1972,11: 705-713.
[19] Dreyer D L. Chemistry and Chemical Taxonomy of the Rutales. London: Academic Press,
1983.
[20] Champagne D E, Koul O, Isman M B et al. Phytochemistry, 1992, 31: 377-394.
[21] Serit M, Ishida M and Kim M. Agric. Biol. Chem., 1991, 55 (9):2381-2385.
[22] Vanucci C, Lange C and Lhommet G. Phytochemistry, 1992, 31 (9): 3003-3334.
原春兰 王晓玲 杨得锁
(宝鸡文理学院化学化工系 陕西宝鸡721007)
摘要 经系统溶剂分离、制备TLC和光谱分析,小叶碟添加法对分离产物进行监测,白藓皮中主要的活性物质是梣酮,其对三龄初期粘虫的72h拒食率和死亡率分别为90.4% 和 95.2%。梣酮对7种细菌和10种植物病害的抑菌活性的测定结果表明,其在50ppm时对大肠杆菌、金黄色葡萄球菌、巨大芽孢杆菌的抑菌率分别为100%, 99.8% 及 99.8%,在100ppm时对小麦纹枯病和黄瓜炭疽病的抑菌率分别为100%和72%。
关键词 白藓皮 梣酮 杀虫活性 抑菌活性