http://www.chemistrymag.org/cji/2006/081006pe.htm |
Jan. 10, 2006 Vol.8 No.1 P.6 Copyright |
Hydrotalcite-supported palladium for reductive coupling of haloaryls to biaryls
Zhuo Guanglan 1, Jiang Xuanzhen 2, Zhang Bo 3, Ge Zhonghua 3(1Department of Chemistry, Zhejiang Sci. & Tech. University, Hangzhou, Zhejiang, 310018; 2Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang, 310027; 3Department of Chemical Engineering , Zhejiang University of Technology, Hangzhou, Zhejiang, 310014) Received on Nov. 28, 2005; Supported by the National Natural Science Foundation of China(No. 20476092) and Open Foundation of National Key-Lab of Green chemistry.
Abstract A new and efficient
heterogeneous catalyst based on palladium particles supported on hydrotalcite was
developed for reductive coupling of chloro- and bromo-aryls to biaryls. The catalyst was
reusable without obvious loss in catalytic activity.
Keywords palladium; hydrotalcite; reductive coupling; biaryls; haloaryls
1. INTRODUCTION
Reductive coupling of aromatic halides is a well-known synthesis tool for C-C bond
formation. The coupling products are found numerous applications in the preparation of
agrochemicals, pharmaceuticals and natural products. It offers a mild alternative to the
classical Ullmann coupling, which required a stoichiometric amount of copper and very high
temperature. The reductive coupling reaction is often catalyzed by palladium, based on the
Pd2+→Pd0 redox cycle.
Various palladium-catalyzed biaryls synthesis are described in the literature [2-7]. In order to in situ regenerating the active palladium catalyst, different reductive reagents, e.g. 2-propanol, hydrogen gas, zinc, or aqueous alkali formate salts, have been attempted. Despite of its feasibility, however, the homogeneous palladium catalyst suffer from the difficulty of reusing. Therefore, heterogeneous catalyst systems are highly desirable. Recently, Sasson and co-workers investigated Pd/C catalyzed homocouplings of aryl chlorides in detail [8-12]. They demonstrated that Pd/C is a very effective catalyst for this coupling reaction. Furthermore, they found that supported PTC ( phase transfer catalyst) is a selective agent for biphenyl synthesis, meanwhile it can suppress the side reaction.
On the other hand, hydrotalcite like anionic clays (HTLC) have received much attention in recent years because it is regarded as an environment-friendly solid base catalyst. Hydrotalcite -supported palladium catalyst has been used in many important reactions such as the oxidation of alcohols[13,14]. Recently palladium modified hydrotalcite was found to be an effective catalyst for Heck, Suzuki, Sonogashira and Stille type coupling reactions[15,16]. This encourages us to apply Pd-hydrotalcite catalyst (Pd/HT) on the reductive coupling reaction. To our knowledge, this is the first report of Pd-HT used as a heterogeneous catalyst for reductive coupling reaction. 2. RESULTS AND DISCUSSION
The diffraction peaks of sample which was prepared in this study can be indexed by the JCPDS X-ray powder diffraction file of No.22-700 as shown in Figure 1. It indicated that the sample was a typical hydrotalcite-supported palladium.
Various supported catalysts, such as Pd/C, Pd/g-Al2O3, Pd/KF/g-Al2O3 and Pd/HT etc. were examined in reductive coupling reaction of chlorobenzene. The experimental results were given in Table 1. It can be seen therein that, surprisingly, 10wt%Pd/HT exhibits the highest catalytic activity among them with 85% yield of biphenyl product and 95% selectivity (Entry 5). However the commercial available 10%Pd/C catalyst shows moderate reactivity (Entry 3) which is quite consistent with the results in literature[8,9]. The rest catalysts demonstrate relatively low catalytic activities(Entries1 and 2) compared with Pd-HT and Pd/C catalysts. However when unreduced Pd2+/HT catalyst was used as the catalyst for reductive coupling, even for longer reaction time (12 h), no reaction takes place at all (Entry 6). It maybe attributed to its heterogeneous nature because homogeneous Pd(OAc)2 and PdCl2(PhCN)2 catalysts are able to catalyze the reductive coupling of aryl and heteroaryl halides as reported by Hassan and Tanaka [2,6].
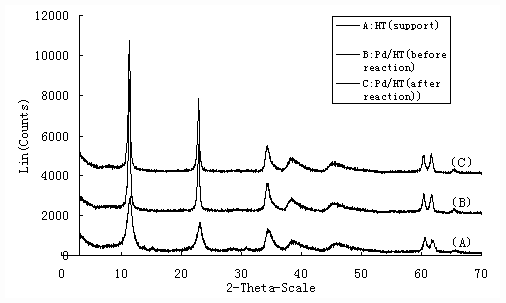
Figure 1. X-ray diffraction pattern of samples with 10%Pd/HT
Table 1 Reaction performances of reductive coupling of chlorobenzene catalyzed by different catalysts
Entry |
Catalyst |
Yield (%) |
Selectivity (%) |
1 |
Pd/g-Al2O3 |
43 |
75 |
2 |
Pd/KF/g-Al2O3 |
55 |
78 |
3 |
Pd/C |
70 |
80 |
4 |
Pd/C a |
25 |
48 |
5 |
Pd/HT |
65 |
82 |
6 |
Pd2+/HT |
none |
none |
a. In the absence of TBAB The influence of phase transfer catalyst (PTC, e.g. TBAB) on the reaction performence was also examined as presented in Entries 3 and 4 of Table 1. The yield of biaryl product sharply reduced when TBAB was absent (see Entry 4), it is well-known that supported palladium catalysts are very active for hydrodehalogenation, which results in the formation of benzyl derivatives. Therefore there exists a competitive reaction between hydrodehalogenation and coupling reaction. The role of PTC has been previously investigated [10,11], it prevents the hydrodehalogenation reaction and promotes the selectivity towards the aryl-aryl coupling direction.
Various aromatic halogenide substrates were performed in reductive coupling reactions (Scheme1), and the results were listed in Table 2. It can be seen from Table 2 that chlorobenzene and its derivatives could be converted to the corresponding biaryl products with moderate to good yields and high selectivities. It is worthwhile to note KOH seems to be a more suitable base in this reaction. But the m-amino chlorobenzene yields no coupling product. We also try to enlarge the application scope. But the results are disappointed. When o-chloro or o-bromo pyridine were selected as the substrate, the reaction seemed to turn to the hydrodehalogenation and the main product was pyridine, only small amount of bipyridine (<20%) were obtained. When chlorobenzene and phenol were selected as the substrates, no cross coupling products were formed but biphenyl with 35% yield.
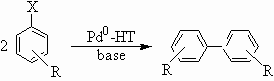
X= Br, Cl
R= H, Me, OH, NH2
Scheme 1 Table 2. Reductive coupling of aromatic halides over Pd0-HT heterogeneous catalyst
Entry |
Substrate |
Product |
Yield |
Selectivity |
|
X |
R |
||||
1 |
Br |
H |
biphenyl |
70 |
80 |
2 |
Cl |
H |
biphenyl |
65 |
82 |
3 |
Cl |
H(a) |
biphenyl |
85 |
95 |
4 |
Cl |
o-Me |
2,2'-dimethyl biphenyl |
25 |
64 |
5 |
Cl |
p-Me |
4,4 '-dimethyl biphenyl |
63 |
80 |
6 |
Cl |
m-NH2 |
- |
0 |
- |
7 |
Br |
o-Me |
2,2 '-dimethyl biphenyl |
45 |
75 |
8 |
Br |
p-Me |
4,4 '-dimethyl biphenyl |
72 |
85 |
9 |
Cl |
p-OH |
4,4 '-dihydroxy biphenyl |
60 |
70 |
a: KOH was used instead of NaOH as the base. As regards catalyst recycling , after simple filtration and washing with methanol, the recovered Pd/HT catalyst could be reused at least three times, no obvious deactivation was observed as presented in Table 3.
Table 3. The recycling performance of Pd/HT in reductive coupling of chlorobenzene
No. |
1st run |
2nd run |
3rd run |
4th run |
Yield (%) |
85 |
82 |
77 |
80 |
Selectivity (%) |
95 |
95 |
92 |
92 |
In conclusion, Pd0-hydrotalcite is the efficient heterogeneous catalyst for reductive coupling reaction. Unlike the homogeneous catalysts, it could be reusable without obviously losing catalytic activity. This makes it to be a suitable candidate for C-C bond formation reactions.
3. EXPERIMENTAL
3.1 Preparation of Pd0-hydrotalcite ( Pd/HT) catalyst
A solution of 0.49 mol of NaOH and 0.1 mol of Na2CO3 in 200 ml of
water was added dropwise to a solution of 0.15 mol of Mg(NO3)2·6H2O
and 0.05 mol of Al(NO3)3·9H2O in 75 ml of water
over a period of 1 h with vigorous stirring at room temperature. After that, an aqueous
solution of H2PdCl4 (0.01188g Pd/ml) was slowly added with stirring.
The final pH was kept above 10. The solution was then heated at 80
3.2 General procedure for reductive coupling of aromatic halides
A 100 ml flask reactor was charged with 0.03 mol of aromatic halides, 0.03 mol of sodium formate, 0.03 mol of NaOH 0.05 mol of TBAB, 0.2 g Pd0-HT, 5 ml water and 15 ml of methanol. The mixture was stirred and heated to reflux (ca. 65ºC) for 2 h, cooled to room temperature and diluted with water. The catalyst was filtered. The organic compounds were extracted with CH2Cl2 (2×20 ml). The combined filtrates were evaporated to give the products. The products were analyzed by GC and identified by GC-MS and FT-IR. REFERENCES
[1] Littke A D, Fu G C. Angew. Chem. Int. Ed. 2002,41:4176.
[2] Hassan J., Penalva V., Lavenot L. etal. Tetrahedron, 1998,54:13793.
[3] Mukhopadhya S, Ratner S, Spernat A et al. Org. Pro. Res. & Dev. 2002,6:297.
[4] Mukhopadhya S, Joshi A V, Peleg L et al. Org. Pro. Res. & Dev. 2003,7:44.
[5] QafishehN., Mukhopadhyay S, Sasson Y. Adv. Synth. Catal. 2002,344:1079.
[6] Kuroboshi M, Waki Y, Tanaka H. J. Org. Chem. 2003,68:3938.
[7] Mukhopadhyay S., Rothenberg G, Qafisheh N et al. Tetrahdron Lett. 2001,42:6117.
[8] Gitis D, Mukhopadhyay S, Rothenberg G et al. Org. Pro. Res. & Dev. 2003,7:109.
[9] Mukhopadhyay S, Yaghmur A., Baidossi M. et al. Org. Pro. Res. & Dev. 2003,7:641.
[10] Mukhopadhyay S, Rothenberg G, Gitis D et al. J. Chem. Soc., Perkin Trans2. 1999,2481.
[11] Mukhopadhyay S, Rothenberg G, Gitis D et al. Org. Lett. 2000,2:211.
[12] Mukhopadhyay S, Rothenberg G, Gitis D et al. J. Org. Chem. 2000,65:3107.
[13] Kakiuchi N, Maeda Y., Nishimura T. et al. J. Org. Chem. 2001,66:6620.
[14] Nishimura T, Kakiuchi N, Inoue M et al. Chem. Commun., 2000,1245.
[15] Choudary B M, Madhi S, Chowdari N S. et al. J. Am. Chem. Soc. 2002,124:14127.
[16] Bennur T H, Ramani A, Bal R et al. Catal. Commun. 2002,3: 493. 水滑石负载钯催化卤代芳烃还原偶联反应研究
卓广澜1, 姜玄珍2 , 张波3 葛忠华3
(1浙江理工大学化学系, 杭州, 310018; 2浙江大学化学系,杭州, 310027; 3浙江工业大学化工学院, 杭州, 310014)
摘要 研究了水滑石负载钯催化氯代和溴代芳烃的还原偶联反应,发现这种催化剂对氯代芳烃还原偶联反应具有较好的反应活性和选择性,较好条件下对氯苯可以达到85%的联苯产率和95%的选择性。反应前后水滑石结构基本不变,催化剂重复使用4次后催化活性基本保持不变。
关键词 钯;水滑石;还原偶联;联苯;卤代芳烃