http://www.chemistrymag.org/cji/2006/081008ne.htm |
Jan. 20, 2006 Vol.8 No.1 P.8 Copyright |
Wang Yu, Fang Zhijie,Jao Yan ,Wang Yuanxing
(Chemical Engineering School, Nanjing University of Science & Technology,
Nanjing,210094)
Supported by the National Natural Science Foundation of China (No.29972020)
Abstract Ab intito Hartree-Fock(HF) and MØller-Plesset second order (MP2) perturbation theories for the correlation energy were applied to the investigation of the thermodynamic properties of 1,4-O-Bis(2,3,4- tri-O-acetyl -
b-D-glucopyranosyloxy)benzene(I)and terephthalyl chloride(II) with 3-21G* basis sets. Cyclic structures were identified as the most stable conformation of the acetylized glycophane(III) , the energy change (DE) in the process of 2 I + 2 IIKeywords Ab initio, glycophane, thermodynamic properties. 1. INTRODUCTION
Glycophane is a new type of neutral, chiral and water-soluble synthetic receptor which have hydrophobic cavities and can be concerned with the carbohydrate recognition processes. The recognition processes can be widely applied not only for the complexation between oligosaccharides and agglutinins or antibodies, but also for the fields of biology, medicine and catalysis etc. Since the synthesis of glycophane is very difficult, with complicated steps, only a few glycophanes have been synthesized in the last 20 years, and have not been in extensive use[1-4], our research group has primarily made lots of the research recently.
A novel synthesis of acetylized glycophane route[5] has been designed, that is the annelation between 1,4-O-Bis (2,3,4-tri-O-acetyl-b-D-glucopyranosyloxy) benzene(I) terephthalyl chloride(II) to synthesize acetylized glycophane(III) as shown in Scheme 1.
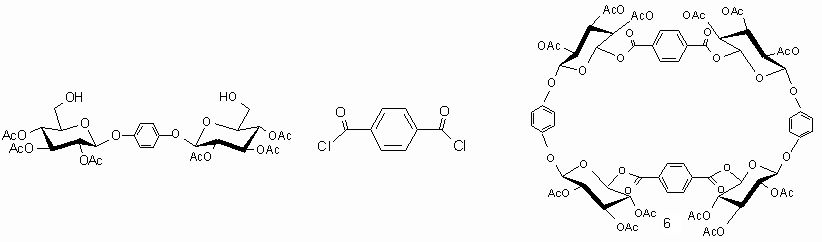
To study the changes of both the total energy and Gibbs free energy concerning the synthesis of such glycophane, either ab initio or semiempirical methods can be employed. Geometric model was primely optimized with PM3 of semi-empirical calculation to give initial conformation for HF/3-21G* calculations.
The energies in different solutions and the changes in thermodynamic properties at different temperatures in the process of synthesizing acetylized glycophane(Ⅲ) were investigated to predict optimal reaction condition[9-12].
All quantum chemical calculations were performed with the Gaussian 98 suite of programs. 3. RESULATS AND DISCUSSION
3.1 Total Energies
Table 1 shows the total energies of three compounds in different solutions at the MP2/3-21G* levels, which indicating that the HF-optimized structure can be used to estimate the binding energies. Table 1 The total energies of three compounds in different solutions at the MP2/STO-3G levels
Energy |
I |
II |
III |
EIII-2EII-2EI-4EHCl |
|
vaccum |
E(HF) |
-61.27 |
-50.49 |
-233.71 |
-10.07 |
E(MP2) |
-65.09 |
-53.18 |
-230.99 |
-11.34 |
|
dichloromethane solvent |
E(HF) |
-53.70 |
-46.04 |
-228.40 |
-28.83 |
E(MP2) |
-55.47 |
-47.26 |
-231.77 |
-27.37 |
|
chloroform solvent |
E(HF) |
-50.89 |
-44.27 |
-207.55 |
-20.21 |
E(MP2) |
-51.47 |
-42.18 |
-205.58 |
-22.57 |
|
acetone solvent |
E(HF) |
-53.44 |
-41.58 |
-198.66 |
-15.52 |
E(MP2) |
-58.09 |
-45.37 |
-189.47 |
-16.82 |
|
The energies for the
compound I, II and III in dichloromethane solution were estimated to be -55.47, -47.26 and
-231.77 kJmol-1 at the MP2/3-21G* level respectively. The changes of the binding energies
in the process of 2 I + 2 II![]() III + 4HCl,
that is the values of the energy change: DE=EIII-2EII-2EI- 4EHCl, are
all negative at different computational levels, The binding energy of compound III is large enough to compensate for the
energies needed for the dissociation of compound I and II. From the view point of energy, the formation of
acetylized glycophane is feasible when the compound I and II are mixed. From the values of the binding energies, it can be
predicted that the compound III might be the easiest
synthesized in the mixture of compound Iand II with dichloromethane or chloroform as solvent, so we can select
dichloromethane or chloroform as solvent to synthesize the acetylized glycophane.
III + 4HCl,
that is the values of the energy change: DE=EIII-2EII-2EI- 4EHCl, are
all negative at different computational levels, The binding energy of compound III is large enough to compensate for the
energies needed for the dissociation of compound I and II. From the view point of energy, the formation of
acetylized glycophane is feasible when the compound I and II are mixed. From the values of the binding energies, it can be
predicted that the compound III might be the easiest
synthesized in the mixture of compound Iand II with dichloromethane or chloroform as solvent, so we can select
dichloromethane or chloroform as solvent to synthesize the acetylized glycophane.
3.2 Thermodynamic Properties
On the basis of vibrational analysis and statistical thermodynamic study, the standard
thermodynamic functions, entropies(Smo) and enthalpies (Hmo),
were obtained and listed in Table 2.
In the process of 2 I + 2 II![]() III + 4HCl ,values
of DST
and DHT are positive and negative respectively, and both the values
fluctuates as the temperature increases from 258.15 to 298.15K. The process is therefore
an exothermic process accompanied by an increase in entropy. From the equation DG=DH-TDS, the change in
Gibbs free energy (DG) in the process was predicted to be -70.01kJmol-1 at 273.15K, the acetylized glycophane(III)
can be spontaneously produced, which is also in agreement with the experimental fact that
the acetylized glycophane can relatively easily be synthesized at 273.15K than the case at
other temperature.
III + 4HCl ,values
of DST
and DHT are positive and negative respectively, and both the values
fluctuates as the temperature increases from 258.15 to 298.15K. The process is therefore
an exothermic process accompanied by an increase in entropy. From the equation DG=DH-TDS, the change in
Gibbs free energy (DG) in the process was predicted to be -70.01kJmol-1 at 273.15K, the acetylized glycophane(III)
can be spontaneously produced, which is also in agreement with the experimental fact that
the acetylized glycophane can relatively easily be synthesized at 273.15K than the case at
other temperature.
Table 2 Thermodynamic properties of the three compounds at the MP2/3-21G* level.
I |
II |
III |
2 I + 2 II |
|
| T(K) | Smo
Hmo |
Smo
Hmo |
Smo
Hmo |
△ST
△HT △GT |
258.15 |
584.73 47.77 |
513.25 40.28 |
2271.55 153.03 |
43.27 -39.52 -50.69 |
273.15 |
612.55 67.31 |
529.09 43.69 |
2289.57 170.27 |
68.70 -51.24 -70.01 |
288.15 |
640.47 80.05 |
545. 61 57.80 |
2318.75 216.68 |
23.33 -55.42 -62.14 |
298.15 |
653.76 95.22 |
559.20 70.56 |
2388.70 257.77 |
30.12 -50.22 -59.32 |
| DST= (Smo)III+4(Smo)HCl -2(Smo)I-2(Smo)II; DHT= (Hmo)III+4(Hmo)HCl -2(Hmo)Ⅰ-2(Hmo)II; DGT=DHT-TDST | ||||
4. CONCLUSIONS
The ab initio quantum chemistry
calculations on 1,4-O-Bis (2,3,4- tri-O-acetyl-b-D-glucopyranosyloxy)
benzene (I), terephthalyl chloride(II) and acetylized glycophane (III) shows, that the
total energy change and the change in Gibbs free energy provide theoretical judgements and
dichloromethane prediction that the formation of the compound (III) with the compound
(II) and (I) as intermediates in dichloromethane or chloroform solvent at 273.15K
might be the easiest, which is consonant with the experiments.
The authors thank the National Natural Science Foundation of China for financial support (No.29972020), and thank Prof. Xue Hai Ju for his beneficial suggestions. REFERENCES
[1] Penades S, Morales J C et al., J Org Chem, 1998,63(25), 9212-92222.
[2] Morales J C, Penades S, Tetrahedrom Lett, 1996,67(28), 5011-5014.
[3] Morales J C, Penades S, Angew Chem Int Ed Engl, 1998,37, 654-657.
[4] Junquera E, Laynez J, J Org Chem, 1996, 61, 6790-6798.
[5] Wang Y, Fang Z J, Wang Y X, Chem J Int, 2004, 6(12), 91-93.
[6] Hobza P, Zabrdnik R, Chem Rev, 1988, 88, 871-897.
[7] Hobza P, Sclzlc H L, Schlag E W, Chem Rev, 1994, 94 ,1767-1785.
[8] Chalasinski G, Szczesniak M M, Chem Rev, 2000,100, 4227-4252.
[9] Raghavachari K, Pople J A, Replogic E S et al., J Phys Chem, 1990, 94, 5579-5586.
[10] Mcdouall J J, Peasley K, Robb M A, Chem Phys Lett, 1988,148, 183-189.
[11] Binkley J S, Pople J A, Int J Quantum Chem, 1975, 9, 229-236.
[12] Ju X H, Xie L J, Hua W J et al., J Phys Org Chem, 2004, 17, 113-117.
糖番前体---1,4-O-二(2,3,4-三-O-乙酰基-
b-D-吡喃葡萄糖苷)苯的溶剂化效应及其热力学性质的理论研究王煜,方志杰*,焦岩,王远兴
(南京理工大学化工学院,南京,210094)
摘要 以从头算法Hartree-Fock(HF) 以及 M?ller-Plesset 第二等级(MP2)的相关能微扰理论为基础,采用3-21G*基元组对1,4-O-二(2,3,4-三- O -乙酰基-β-D-吡喃葡萄糖苷)苯(Ⅰ)及对苯二甲酰氯(Ⅱ)的热力学性质进行了模拟计算研究。乙酰化糖番(Ⅲ)的环状结构很稳定,以二氯甲烷为溶剂的 2 I + 2 II
关键词 从头算法,糖番,热力学性质