http://www.chemistrymag.org/cji/2006/082012ne.htm |
Feb.12, 2006 Vol.8 No.2 P.12 Copyright |
Sui Chaoxia1, Yang Fang1,2,
Zheng Wenjie1,2, Bai Yan1
(1 Department of Chemistry, Jinan University; 2 Research Center of
Hydrobiology, Jinan University, 510632, China)
Abstract A series of complexes CuL2Cl2
(L= NS, MB, BS) were synthesized, based on the liquid-phase reaction of piazselenole(NS),
5-methyl- piazselenole(MB), 4,5-benzopiazselenole(BS), which acted as ligands and Cu2+
as cation. The physicochemical properties of the complexes were determined by
ICP-AES, EA, FT-IR analysis, UV-Vis, XRD, FS etc. The coordination mechanism between
ligands and Cu2+ was also discussed.
Keywords heterocyclic aromatic selenium compounds, copper complexes, synthesis and
characterization
Selenium is the trace element controlled by gene in human body[1]. Heterocyclic aromatic selenium compounds (Piaselenole, Pis) are a sort of important compounds derived by the reaction between aromatic ortho-aromatic diamine and selenium dioxide. Pis have attracted considerable interests in many fields, such as new functional materials, analytical reagents and new drugs etc[2-6]. Copper is also an essential element in vivo, and it is also sulphophile element. A series of complicated reactions take place between copper and sulphur (selenium) in the metabolism. However, the biochemistry mechanism and the reciprocity of the reaction between selenium and copper is not clear at the present time[7]. Consequently, the coordination mechanism between Cu2+ and the small molecule of selenium compounds, especially heterocyclic aromatic selenium compounds will be helpful to explain metabolic mechanisms of copper and selenium, which will be significant to develop new drugs and new functional materials with selenium.
1 EXPERIMENTAL
1.1 Reagents and chemicals
o-phenylenediamine, 3,4-Diaminotoluene, 2,3-Diaminonaphthale, all ortho-aromatic
diamines were purchased from Sigma and used without any further purification, CuCl2(anhydrous),
SeO2, anhydrous ethanol, ethanol (95%).
1.2 Synthesis of ligands
The ligands were synthesized according to literature [4]. The ligands
synthesized in the article included: 2,1,3-napheno[3,4-c]selenadiazole (BS),
5-methy-2,1,3-benzo[3,4-c]selenadiazole( MB), 2,1,3-benzo[c]selenadiazole (NS), the
structural formula were as follows:



NS MB BS
1.3 Synthesis of CuL2Cl2 complexes
The complexes CuL2Cl2 (L= BS/ MB/ NS) were synthesized by
modified methods in literature [8]. The mole ratio of ligands to CuCl2
was 7:1. The ligands and CuCl2 were dissolved in anhydrous ethanol separately,
then mixed and stirred for 1.5h at 55℃, and a great
deal of precipitate came into being while stirring. The precipitate was filtered quickly
and washed several times with ethanol (95%).
1.4 Characterization of CuL2Cl2 complexes
The contents of Se and Cu were analyzed by Inductively Coupled Plasma-Atomic Emission
Spectrometry (ICP-AES) on Optime 2000 DV spectrometer (Perkin-Elmer company, America). The
contents of C, H, N were determined by Vario EL spectrometer (ELEMENTAR company, Germany).
FT-IR spectra of complexes were taken with potassium bromide pellets on a Equinox55
spectrometer. Scan range: 400-4000 cm-1. UV-Vis spectra were recorded on a
UV-260 spectrophotometer in N,N-dimethylformamide
solution. Fluorescence spectra were recorded on a 970CRT fluorescent spectrophotometer
(Shanghai SANCO Instrument Co. Ltd) in N,N-dimethylformamide
solution. X-ray diffraction patterns were recorded on a XD-98 diffractometer equipped with
Cu-anode, Radiation of Cu Ka1 (0.15405981nm), Power setting of 40KV, 20mA. Scan
speed of 5s/step, Divergence slits of 1 deg., Receiving slits of 0.15mm, Soller slits of
1. All experiments were carried out at room temperature.
2 RESULTS AND DISCUSSION
2.1 Composition analysis
Elemental analysis (EA) results of the complexes were given in Table 1. The
productivity and color of the complexes were given in Table 2, FT-IR spectra. UV-Vis
spectra and FS spectra for ligands and complexes were shown in Table. 3-4. X-ray
diffraction patterns and crystal degree were shown in Fig. 1 and Table 5 respectively.
Table 1 Elemental analysis results of complexes (%)
complexes |
C |
H |
N |
Cu2+ |
Se |
Cu( NS)2Cl2 |
28.92(28.75)* |
1.66(1.60) |
11.36(11.18) |
12.56(12.67) |
31.74(31.54) |
Cu(MB)2Cl2 |
31.19(31.76) |
2.24(2.27) |
10.54(10.59) |
12.03(12.01) |
29.50(29.87) |
Cu(BS)2Cl2 |
40.80(39.97) |
1.88(1.99) |
9.21(9.33) |
10.51(10.58) |
26.15(26.30) |
﹡The theoretical value is in bracket.
The content of element calculated from the results of elemental analysis was consistent with the theoretical value of CuL2Cl2 according to Table 1, which indicated that reaction between ligands and Cu2+ was complete and the CuL2Cl2 complexes were synthesized.
Table2 The productivity and color of the complexes
Cu( NS)2Cl2 |
Cu(MB)2Cl2 |
Cu(BS)2Cl2 |
|
Color |
Brilliant green |
Yellow green |
Purple black |
Productivity |
55.45 |
32.48 |
50.36 |
Table 3 FT-IR data of ligands and complexes (cm-1)
Compounds |
n (Ar-N-) |
n (-C-H) | Vibration of framework of aromatic ring |
n (N-Se-N)[9,10] |
NS |
3448s |
2923w, 2855w |
1633s |
442w, 488w, 744s |
Cu (NS)2Cl2 |
3442s |
2925w, 2854w |
1632s |
498w, 579w,729w |
MB |
3448s |
2913s, 2858w |
1626vs |
498w, 574s, 708w |
Cu (MB)2 Cl2 |
3437s |
2926w, 2857w |
1632s |
499w, 572w, 798s |
BS |
3445s |
2925w, 2854w |
1633s |
493w, 530w |
Cu (BS)2 Cl2 |
3447s |
2923s, 2853w |
1681w, 1636s |
490w, 594s, 709w |
As seen in Table 3, a strong stretching peak appeared at 3450 cm-1 which attributed to Ar-N- group in the ligands. The peaks had red shift or blue shift to a certain degree after the coordination. There were hardly change at 2850~2950cm-1, which indicated that framework of aromatic rings had no effect on the coordinate reaction of Se and Cu. There were obvious differences about the peaks at 490~750cm-1 attributed to N-Se-N group between the complexes and the ligands, which indicated that N-Se-N group was the coordinate site of ligand and Cu2+.
Table 4 Data of UV-Vis spectra for ligands and complexes
Compounds |
lmax/n(e/104) |
||||||
NS |
222(0.31) |
238(0.21) |
254(2.64) |
262(0.94) |
332(1.17) |
||
Cu( NS)2Cl2 |
224(0.30) |
236(0.35) |
240(0.36) |
256(0.48) | 265(1.41) | 332(1.12) |
|
MB |
233(2.02) |
239(0.91) |
243(0.68) |
264(0.82) |
336(0.76) |
||
Cu(MB)2Cl2 |
237(1.02) |
264(1.29) |
335(0.50) |
||||
BS |
227(0.226) |
236(0.23) |
240(0.22) |
248(0.23) |
265(0.81) |
379(0.31) |
|
Cu (BS)2Cl2 |
222(0.75) |
230(0.80) |
266(3.82) |
368(1.34) |
379(2.13) |
The UV-Vis spectra of ligands and complexes were shown in Table 4, and there were obvious differences between the complexes and the ligands. Each of the characteristic peak had red shift or blue shift to a certain degree, and some of them disappeared and new peaks appeared after the coordination. Absorption peaks of BS at 240, 248nm disappeared and absorption peaks shifted from 227, 236nm to 222, 230nm respectively, while new absorption peaks appeared at 368nm after the coordination. Similarly new absorption peaks appeared at 240nm and the peak at 222nm had blue shift in NS. Absorption peaks at 233, 243nm disappeared in MB after the coordination. The coordinate effect was the reason of changes in the UV-Vis spectra.
Table 5 Data of Fluorescence spectra for the ligands and complexes
Compounds |
concentration |
Ex (nm) |
Em (nm) |
F |
NS |
2.2 |
— |
— |
— |
Cu( NS)2Cl2 |
2.2 |
— |
— |
— |
MB |
2.0 |
380 |
434 |
486.24 |
Cu(MB)2Cl2 |
2.0 |
380 |
434 |
622.30 |
BS |
1.9 |
378 |
580 |
905.57 |
Cu(BS)2Cl2 |
1.9 |
378 |
573 |
448.09 |
As seen in
Table 5, NS had no characteristic fluorescence and BS had intensive fluorescence peak at
room temperature, as was described in the literature [11]. After the
coordination between ligands and CuCl2, NS still had no fluorescence spectra,
while fluorescence intensity of MB and BS had great changes and the peak position had no
change, which indicated that the luminescence of complex was the ligand. It seemed as if
fluorescence intensity of MB increased after coordinating with CuCl2, but
actually it decreased because the ligand concentration in the complex was two times of the
ligand. Fluorescence intensity obviously dropped after coordination with CuCl2.
The fluorescence data indicated that CuCl2 could induce fluorescence quenching
of complex after coordinating with ligands.
It can be seen from the above analysis that molecular orbital energy
level of the ligands had great changes after the coordination between heterocyclic
aromatic selenium compounds and CuCl2, which caused the change of
intramolecular electron transition energy of the ligands and resulted in the absorption
changes of the complexes.
2.2 X-ray diffraction
X-ray diffraction of ligands, CuCl2
and complexes were shown in Fig. 1. It could be seen from Fig. 1 that X-ray diffraction of
the complexes was different from the ligands and CuCl2 obviously. The chief diffraction peaks of the reactants disappeared and new
diffraction peaks appeared in the patterns of complexes, which indicated that new compound
was formed.
The
crystal diffraction and the non-crystal diffraction were identified by using the multiple
separation method in computer, then the crystal degree of ligands and complexes were
calculated and the results were given in Table 6. As seen in Table 6, crystal degree of
the ligands has descended obviously after coordinating with CuCl2.
Table 6 Crystal degree of ligands and complexes (crystal degreeχc% )
BS |
Cu (BS)2Cl2 |
MB |
Cu(MB)2Cl2 |
NS |
Cu( NS)2Cl2 |
CuCl2 |
42.3 |
11.3 |
32.0 |
19.3 |
46.3 |
23.7 |
25.6 |
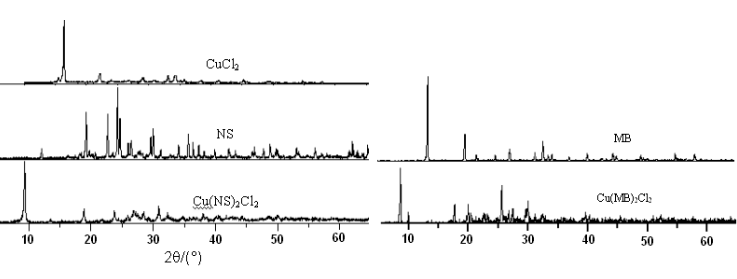
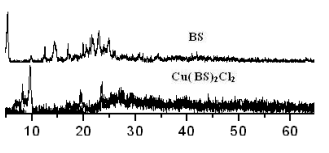
Fig. 1 XRD patterns of complexes CuL2Cl2 ligands and CuCl2
3. DISCUSSION OF COORDINATION MECHANISM
Molecular formula of the complex was CuL2Cl2
(L= NS / MB/ BS), which was the result of reaction between heterocyclic aromatic selenium
compounds and CuCl2. Valence shell electron configuration of the central ion Cu2+
is 3d94s04p04d0, which can form quadridentate (sp3) or sexadentate (sp3d2)
complexes.

Fig. 2 The possible coordinate formula of the complex
Three ligands all had structure of attribute. Two
nitrogen atoms of the structure adopted sp3 hybridization. One hybrid orbital
accommodated the lone pair on nitrogen. The bonding of the selenium atom was various and
sophisticated because it had d orbital. Selenium atom could use sp2
hybridization if not considering d orbital, so a pair of p electrons had a share in
conjugate and lone electron pair hold one sp2 hybrid orbital. Consequently, two
nitrogen atoms and one selenium atom all had lone electron pair which could coordinate
with CuCl2. In addition, the electron-rich five member cycloalkane could
coordinate with CuCl2. That is to say, the site of coordination where three
ligands coordinated with CuCl2 was the five member cycloalkane. The above
deduction had been proved by IR and UV-Vis. Further considering configuration and
geometric of the ligands as well as lone electron pair space orientation of hetero-atom,
it can be seen that the coordinated lone pair of nitrogen atom had great influences on the
steric hindrance originating from the C-H bond of 4-location and 5-loaction. However,
there is little probability of coordination of the five member cycloalkane. So it was most
likely that the coordination site was Cu2+ and selenium atom. As to the
complexes CuL2Cl2 (L= NS / MB/ BS), copper was quadridentate sp3
or sexadentate sp3d2 depending on whether chloride ion formed bridge
bond or not. When chloride ion coordinated through bridge bond, the complex could form
chain structure. In this structure, copper ion formed sp3d2 hybridization
utilizing outer-shell d orbital; the four chloride ions and copper ion was in the same
plane, and the two ligands were vertical to the plane and located just above and under the
plane. The structural formula of the complex was shown in Fig. 2.
REFERENCES
attribute. Two
nitrogen atoms of the structure adopted sp3 hybridization. One hybrid orbital
accommodated the lone pair on nitrogen. The bonding of the selenium atom was various and
sophisticated because it had d orbital. Selenium atom could use sp2
hybridization if not considering d orbital, so a pair of p electrons had a share in
conjugate and lone electron pair hold one sp2 hybrid orbital. Consequently, two
nitrogen atoms and one selenium atom all had lone electron pair which could coordinate
with CuCl2. In addition, the electron-rich five member cycloalkane could
coordinate with CuCl2. That is to say, the site of coordination where three
ligands coordinated with CuCl2 was the five member cycloalkane. The above
deduction had been proved by IR and UV-Vis. Further considering configuration and
geometric of the ligands as well as lone electron pair space orientation of hetero-atom,
it can be seen that the coordinated lone pair of nitrogen atom had great influences on the
steric hindrance originating from the C-H bond of 4-location and 5-loaction. However,
there is little probability of coordination of the five member cycloalkane. So it was most
likely that the coordination site was Cu2+ and selenium atom. As to the
complexes CuL2Cl2 (L= NS / MB/ BS), copper was quadridentate sp3
or sexadentate sp3d2 depending on whether chloride ion formed bridge
bond or not. When chloride ion coordinated through bridge bond, the complex could form
chain structure. In this structure, copper ion formed sp3d2 hybridization
utilizing outer-shell d orbital; the four chloride ions and copper ion was in the same
plane, and the two ligands were vertical to the plane and located just above and under the
plane. The structural formula of the complex was shown in Fig. 2.
REFERENCES [1] Zheng W J, Ouyang Z. Organic selenium compounds from plants: their chemistry & applications in medicine(ZhiWu YouJi Xi De HuaXue JiQi YiXue YingYong), Jinan University Press, Guangzhou, 2001.
[2] Zhang J L, Zou J H, Zheng W J et al. Chemical Research and Application(HuaXue YanJiu Yu YingYong), 2004, 16 (4) : 561-562.
[3] Xu H B, Huang K X. Selenium: Its Chemistry, Biochemistry and Application in Life Science(Xe De HuaXue ShengWu HuaXue JiQi Zai ShengMing KeXue Zhong De YingYong), Huazhong University of Technology Press, Wuhan, 1994.
[4] Zou J H, Yang F, Zheng W J et al. Chemical Reagents(HuaXue ShiJi), 2004, 26 (5): 289-290, 292.
[5] Zhou Y M, Xin XQ, Joutnal of Inorganic Chemistry(WuJi HuaXue XueBao), 1999, 15 (3): 273-292.
[6] Guo L, Yun L H, Chinese Journal of New Drugs(ZhongGuo XinYaoWu ZaZhi), 2000, 9(3): 155-158.
[7] Wang K, Xu H B, Tang R H et al. Trace Element in Life Sicence(ShengMing KeXue Zhong De WeiLiang YuanSu). Zhong Guo Ji Liang Press. BeiJing, 1991.
[8] Richard Harlan Hanson. 1966. B.S. Mankato. State College Doctor of Phlosophy thesis[D].
[9] Kwiatkowski J S, Leszczynski J, Teca I, Journal of molecular structure, 1997, 436-437: 451-480.
[10] Liu Q F, Zhang D T, Wang X L et al. Chinese Journal of Spctroscopy Laboratory(GuangP ShiYanShi), 2003, 20(6): 845-847.
[11] Zheng W J, Zeng Xinhua, Guo B J et al. Spectroscopy and Spectral Analysis(GuangPuXue Yu GuangPu FenXi). 2004, 24(11): 102-105.
硒芳香杂环
-铜配合物的合成与表征睢超霞1 杨芳1,2 郑文杰1,2 白燕1
(暨南大学化学系, 暨南大学水生生物研究所, 广州 610632)
摘要 以硒芳香杂环化合物2,1,3-萘并[c]硒二唑(BS)、5-甲基-2,1,3-苯并[3,4-c]硒二唑(MB)和2,1,3-苯并[c]硒二唑(NS)为配体,采用液相合成法合成了CuL2Cl2 (L= BS、MB和NS)配合物,通过X-射线衍射(XRD)、等离子体原子发射光谱 (ICP-AES)、元素分析(EA)、红外光谱(IR)、紫外可见光谱(UV-Vis)、荧光光谱(FS)和电导率等手段对配合物进行了表征,并初步探讨了配体和Cu2+离子的配位机理。
关键词 硒芳香杂环、 铜配合物、 合成与表征