http://www.chemistrymag.org/cji/2006/083017ne.htm |
Mar. 12, 2006 Vol.8 No.3 P.17 Copyright |
Condensation of aryl aldehydes with rhodanine in water media catalyzed by Tween 80
Luo Jinju, Li Yiqun, Zhou Meiyun
(Department of Chemistry, Jinan
University, Guangzhou 510632, China)
Received on Dec.26, 2005; The project was supported by the National Natural Science Foundation of China (20272018) and the Guangdong Natural Science Foundation (04010458, 021166)
Abstract Condensation reactions of
various aryl aldehydes with rhodanine proceeded smoothly in water without any organic
solvents to afford the corresponding products at room temperature in excellent yields. The
procedure presented has the merits of environmentally benign, simple operation, convenient
work-up and moderate to good yields.
Keywords aldehyde, rhodanine, Tween 80, water media, condensation
The rhodanine derivatives have attracted
considerable pharmaceutical interest. The compounds with rhodanine moiety are reported to
have anticonvulsant[1], antibacterial[2], antiviral[3]
and anti-diabetic[4] properties. Therefore, the preparation of this
heterocyclic core unit has attracted the attention of many organic chemists. The simple
and direct method involves the Knoevenagel condensation of a variety of aryl aldehydes and
rhodanine in refluxing glacial acetic acid[5], ethanol[6], and
toluene[7] in the presence of catalyst. Recently, many other methods including
microwave irradiation[8], dry reaction[9] with high temperature, and
solid phase synthesis[10] also have been employed for this transformation.
However, many of these methods suffer from one or other limitations such as requiring
harsh conditions, low to moderate yields, relatively long reaction time and cumbersome
experimental process. So it is necessary to find a new catalyst for this important
preparation.
Reactions performed in water media have gained much interest in
synthetic chemistry over the past decade, not only for the advantages accorded by avoiding
using and disposal of harmful organic solvents, but also for the unique patterns of
reactivity and selectivity in certain cases compared to the convenient organic solvents[11]. However, the use of water in organic reaction processes
is rather limited because many organic compounds are hydrophobic and many reagents are
sensitive to water, therefore, there is need for the
use of surfactants or water stable catalysts to overcome the barriers. The common used
surfactants are quaternary ammonium cationic compounds such as cetyltrimethylammonium
chloride (CTAC), cetyltrimethylammonium bromide (CTAB) etc.
Unlike the quaternary ammonium salt surfactants, which have significant
toxic effects on a variety of bacteria and fungi[12], Tween 80, (also known as
Polysorbate 80), is safe, biodegradable and found extensive use as a surfactant and
solubilizing agent, not only in chemical, biochemical, pharmacological, and medicine
research, but also in food, cosmetics, and pharmaceutical industries.
In the course of our investigations to develop new synthetic reactions
in water media, we herein report the use of Tween 80 as a catalyst for the condensation of
aryl aldehydes with rhodanine in water media at ambient temperature (Scheme 1).
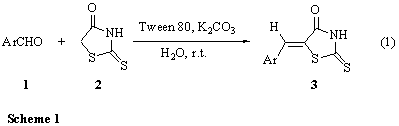
Various aryl aldehydes reacted well with
rhodanine in the presence of a catalytic amount of Tween 80 in potassium carbonate
solution to give the corresponding products in 60-95% yields at room temperature. The
results were summarized in Table 1.
Table 1 Condensation of aryl aldehydes and rhodanine
using Tween 80 as catalyst in water media
entry |
aldehyde (1) |
product (3) |
time (h) |
yielda (%) |
mp (¡ãC) |
|
found |
reported |
|||||
1 |
4-NO2-C6H4CHO |
3a |
3 |
95 |
250.5-253 |
249-250[13] |
2 |
3-NO2-C6H4CHO |
3b |
5 |
87 |
264-265.5 |
263-264.5[8] |
3 |
2, 4-2Cl-C6H3CHO |
3c |
26 |
80 |
234-235.5 |
231.5-232.5[6] |
4 |
2-Cl-C6H4CHO |
3d |
15 |
85 |
189-190 |
192[5a] |
5 |
4-Cl-C6H4CHO |
3e |
30 |
85 |
228.5-229.5 |
231-232[5a] |
6 |
4-MeO-C6H4CHO |
3g |
66 |
60 |
250-252 |
250-251[14] |
7 |
4-Me-C6H4CHO |
3h |
57 |
67 |
221-223 |
219-220[6] |
8 |
C6H5CHO |
3i |
50 |
71 |
204-205.5 |
204.5-206[8] |
9 |
|
3j |
30 |
90 |
231-232 |
228-229[8] |
a
Isolated yield.As shown in Table 1, both the aryl
aldehydes with electron donor groups or withdrawing groups gave the desired products in
moderate to excellent yields in the reaction time ranging from 3 to 66h. The nature of
both electron-withdrawing groups and electron-donating groups on the aromatic ring of the
aldehydes has significant effect on the reaction time and yields. Aryl aldehydes such as p-nitrobenzaldehyde
and m-nitrobenzaldehyde required relatively short reaction time (Table 1, entries
1-2). Aryl aldehydes with electron-donating groups such as p-anisaldehyde and p-methylaldehyde
required much longer reaction time (Table 1, entries 6-7).
In summary, we have described an efficient and eco-friendly procedure
for the condensation reaction of various aryl aldehydes with rhodanine using Tween 80 as
catalyst in water media at ambient temperature with moderate to excellent yields. The
procedure offers several advantages including mild reaction condition, expeditious work-up
condition, simple isolation procedure, cleaner reaction profiles.
Experimental
Melting points were measured by X6 micro-melting point apparatus and were
uncorrected. Infrared spectra were recorded using KBr pellet on a Bruker Equinox 55
spectrometer. 1H NMR spectra were recorded in DMSO-d6 on a Bruker
AVANCE 300£¨300 MHz£©instrument with the residue DMSO as an internal standard at d 2.54
ppm. Furyl aldehyde and benzaldehyde were purified by distillation. All other chemicals
used were of commercial grade without further purification.
General procedure for the condensation of aryl aldehyde and rhodanine: A
mixture of the aryl aldehyde (3 mmol), rhodanine (3 mmol) and K2CO3
(6 mmol) was stirred at ambient temperature in water (10 mL) for the specified time in
Table 1. After completion of the reaction monitored by TLC, the mixture was poured into
water and neutralized with 5% HCl. The precipitant was filtered off and treated with
saturated NaHSO3 and brine to afford the products. Although the products were
found practically pure, further purification was carried out by recrystallization with
aqueous ethanol. All compounds are characterized by melting point, IR, 1H NMR
and also compared with the data reported in the literature.
4-Nitrobenzylidene rhodanine 3a: 1H NMR (DMSO-d6, 300MHz) d :
7.76 (s, 1H, CH=), 7.88 (d, J = 8.82 Hz, 2H, Ar-H), 8.36 (d, J = 8.82 Hz,
2H, Ar-H)£»IR (KBr) n : 3437, 1722, 1640, 1609, 1530,
1446, 1345, 1192 cm-1.
3-Nitrobenzylidene rhodanine 3b: 1H NMR (DMSO-d6,
300MHz) d : 7.79 (s, 1H, Ar-H), 7.81-7.86 (m, 1H, Ar-H), 8.00 (d, J = 7.65 Hz, 1H,
Ar-H), 8.32 (d, J = 8.13 Hz, 1H, Ar-H), 8.44 (s, 1H, CH=)£»IR (KBr) n : 3437, 1702, 1640, 1532, 1407, 1350, 1193 cm-1.
2, 4-Dichlorobenzylidene rhodanine 3c: 1H NMR (DMSO-d6,
300MHz) d : 7.56-7.67 (m, 2H, Ar-H), 7.71 (s, 1H, Ar-H), 7.89 (s, 1H, CH=)£»IR (KBr) n : 3436, 1702, 1639, 1584, 1436, 1196 cm-1.
2-Chlorobenzylidene rhodanine 3d: 1H NMR (DMSO-d6, 300MHz) d
: 7.54-7.58 (m, 3H, Ar-H), 7.66 (m, 2H, Ar-H), 7.46 (s, 1H, CH=)£»IR (KBr) n : 3437, 3074, 1701, 1591, 1435, 1195 cm-1.
4-Chlorobenzylidene rhodanine 3e: 1H NMR (DMSO-d6,
300MHz) d : 7.65 (s, 4H, Ar-H), 7.68 (s, 1H, CH=)£»IR
(KBr) n : 3438, 3085, 1709, 1597, 1442, 1183 cm-1.
4-Methoxylbenzylidene rhodanine 3f: 1H NMR (DMSO-d6,
300MHz) d : 3.04 (s, 3H, CH3), 7.15 (d, J = 8.71 Hz, 2H, Ar-H), 7.60 (d,
J = 8.72 Hz, 2H, Ar-H), 7.65 (s, 1H, CH=)£»IR
(KBr) n : 3437, 1687, 1640, 1586, 1261, 1172 cm-1.
4-Methylbenzylidene rhodanine 3g: 1H NMR (DMSO-d6,
300MHz) d : 2.40 (s, 3H, CH3), 7.40 (d, J = 8.09 Hz, 2H, Ar-H), 7.53 (d,
J = 8.16 Hz, 2H, Ar-H), 7.65 (s, 1H, CH=)£»IR
(KBr) n : 3437, 1693, 1592, 1432, 1235, 1179 cm-1.
4-Benzylidene rhodanine 3h: 1H NMR (DMSO-d6, 300MHz) d :
7.51-7.85 (m, 5H, Ar-H), 7.68 (s, 1H, CH=)£»IR (KBr)
n : 3437, 1702, 1674, 1593, 1438, 1236, 1194 cm-1.
2-Furylidene rhodanine 3i: 1H NMR (DMSO-d6, 300MHz) d : 6.80
(s, 1H, Ar-H), 7.20 (d, J = 3.22 Hz, 1H, Ar-H), 7.51 (s, 1H , Ar-H), 8.13 (s, 1H,
CH=)£»IR (KBr) n : 3438, 3142, 3035, 1690, 1601,
1443, 1319, 1226, 1178 cm-1.
REFERENCES
[1] (a) Troutman H D, Long L M. J. Am. Chem. Soc., 1948, 70: 3436; (b) Mishra S,
Srivastava S K, Srivastava S D. Indian J. Chem., Sect. B, 1997, 36: 826.
[2] Foye W O, Tovivich P, J. Pharm. Sci., 1977, 66: 1607.
[3] Sudo K, Matsumoto Y, Matsushima M et al. Biochem. Biophy. Res. Comm., 1997,
238: 643.
[4] (a) Ohishi Y, Mukai T, Nagahara M et al. Chem. Pharm. Bull., 1990, 38: 1911; (b)
Momose Y, Meguro K, Ikeda H, C. et al. Chem. Pharm. Bull., 1991, 39: 1911.
[5] (a) Campbell N, Mckall J E. J. Chem. Soc., 1948, 1251; (b) Campaigne E, Kreighbaum W
E. J. Org. Chem., 1961, 26: 1326.
[6] Brown F C, Bradsher C K, Bond S M et al. J. Am. Chem. Soc., 1951, 73: 3257.
[7] Giles R G, Lewis N J, Quick J K et al. Tetrahedron, 2000, 56: 4531.
[8] Zhang M, Wang C D, Yu S L et al. Chem. J. Chin. Univ. (Gaodeng Xuexiao Huaxue Xuebao),
1994, 15: 1647.
[9] Li G S, Wang C, Li J C et al. Chin. J. Appl. Chem. (Yingyong Huaxue), 2004, 21:
1069.
[10] Lee C L, Sim M M, Tetrahedron Lett., 2000, 41: 5729.
[11] Li C-J. Chem. Rev., 2005, 105: 3095.
[12] (a) Li G, Shen J, Zhu Y. J. Appl. Polym. Sci., 1998, 67: 1761; (b) Babalola G O.
Lett. Appl. Microbiol., 1998, 26: 43; (c) Kelman D, Kashman Y, Rosenberg E et al. Aquat.
Microb. Ecol., 2001, 24: 9.
[13] Bondzy?ski S, Montash Chem., 1887, 18: 350.
[14] Chakrabariti P M, Chapman N B, Clark K.
Tetrahedron, 1969, 25: 2781.
¡¡