http://www.chemistrymag.org/cji/2006/083018pe.htm |
Mar. 12, 2006 Vol.8 No.3 P.18 Copyright |
Spectrophotometric determination of protein by using Cu(II)-nitrosulfophenol S complex as a spectral probe
Hu Qiuluan a, Wang Chunfengb, Li Quanminc(aDeparment of Chemistry, Luoyang Normal University, Luoyang ,471022, China; bCollege of life Science, Henan Normal University, Xinxiang, Henan, 453007, China; cCollege of Chemistry and Environmental Science, Henan Normal University, Xinxiang, Henan, 453007, China) Received on Jan. 31, 2006; Supported by the Natural Science of Henan's Fundation (0411021600). Abstract A new method for the determination of proteins by using Cu (II)-Nitrosulfophenol S Complex as Spectral Probe was established . This method is based on the binding interaction of protein with Cu (II)-Nitrosulfophenol S Complex in the Clark-Lubs buffer solution at pH1.8-2.2. The binding reaction is complete within 10min at room temperature, and causes absorbance decrease at 617nm of the Cu (II)-Nitrosulfophenol S Complex. The decrease of absorbance is proportional to the concentration of proteins. The linear range for calibration graph of human serum albumin is up to 80mg/L, and the apparent molar absorptivity of binding reaction at 617nm is 4.81×105 L·mol-1·cm-1. The method has been successfully applied to the determination of total amounts of proteins in the human serum samples.
Keywords Cu (II)-Nitrosulfophenol S Complex, Protein, Spectral Probe
1. INTRODUCTION
In the past years, the quantification of
protein in biological samples has drawn great attention as life science and related
research activities move along. Methods for separation and analyzing proteins are booming
up to a great extent, of which the spectrophotometry has been considerably recognized
because it is simple, fast, cost-effective and precise[1-3]. At present, the study for spectrophotometric determination of proteins mainly
concentrates on small molecule such as organic dyes as spectral probe, and there is less
report on the research of the metal complex as spectral probe. Recent researches indicate
that the determination of protein concentration by using metal complex as spectral probe
can obtain higher sensitivity and selectivity [4-6]. In
this paper, the authors for the first time report the binding interaction of protein with
Cu (II)-Nitrosulfophenol S Complex , and designed a new method for the determination of
proteins by using the metal complex as spectral probe. The sensitivity of this method is
higher than most of the spectrophotometric methods which the dyes are directly used as
spectral probe. Many inorganic ions and organic materials do not interfere with the
determination. The method has been applied to the determination of total amounts of
proteins in the human serum samples and satisfactory results are obtained.
2. EXPERIMENTAL
2.1 Reagents
Nitrosulfophenol S (Abbr. R in this paper) was obtained from Beijing chemical plant
(5.0×10-4 mol/L). Bovine serum albumin (BSA), human serum albumin (HSA), g-globulin (g-G), hemoglobin (Hb), egg albumin
(OVA), lysozyme (Lys) were purchased from Sigma. 1.0×10-3mol/L Cu(NO3)2
solution; 0.1mol/L NaCl solution.1g/L glucose solution; 0.5 g/L Citric acid; 1%(V/V)TritonX-100 solution;
100mg/L tetradecylpyridinium bromide(TPB) solution; 100mg/L Sodium dodecyl sulfonate (SDS)
solution; ethanol; pH1.8-2.2 Clark-lubs (C-L) buffer solution was prepared by dissolving
some KHC8H4O4 and some HCl in distilled water and
diluting it to some volume. It was used to control pH values of the test solution. All of the
reagents were of analytical grade, doubly-deionized water used throughout.
2.2 Apparatus
GBC UV/VIS 916 spectrophotometer (GBC Company, Australia) was used for recording the
absorption spectra, and a Model 723 spectrophotometer (Shanghai Jingmi Yiqi Ltd, China)
for the measurement of absorbance at a given wavelength. PHS-3B pH meter (Shanghai Leici)
was used to determine pH values.
2.3 Procedure
The following solutions were added to a 10mL tube:1.5mL C-L buffer solution
(pH2.0),1.0mL Nitrosulfophenol S solution(5.0×10-4mol/L),1.0mL Cu(II)
solution(1.0×10-3mol/L )and HSA standard solution. The mixture was diluted to
10mL with water. After 10min, the absorbance (A0) of Cu (II)-Nitrosulfophenol S
complex solution and the absorbance (A) of Cu (II)-Nitrosulfophenol S-HSA solution were
measured at l=617nm
using water as the blank. DA(A0-A) was calculated.
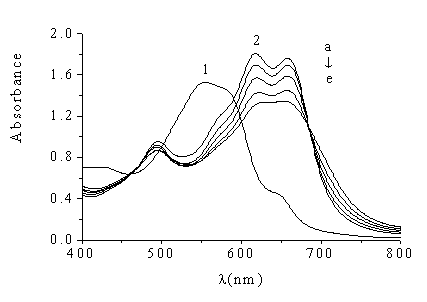
Fig.1 Absorption spectra of
Nitrosulfophenol S ,Cu(II)-Nitrosulfophenol S and Cu(II)-Nitrosulfophenol S-HSA
1 Nitrosulfophenol S (CR=5.0×10-5mol/L )against water; 2. Cu (II)
-Nitrosulfophenol S-HSA (CCu-R=5.0×10-5mol/L )
against water
CHSA (mg/L): a. 0; b. 20; c. 40; d. 60; e. 80
3. RESULTS AND DISCUSSION
3.1 Absorption spectra and reaction mechanism
In the pH2.0 C-L buffer solution, Cu (II)-Nitrosulfophenol S complex solution is blue.
The absorption spectra of Cu (II)-Nitrosulfophenol S complex are shown in Fig.1. 1, with
three absorption peaks, at 495nm, 617nm and 658nm, respectively. After HSA is added, the
fading in color is observed. Two peak values at 617nm and 658nm obviously decrease, and
the decrease of absorbance is proportional to the concentration of proteins (shown in Fig.
1). Based on the above observation, a new method for the spectrophotometric determination
of proteins concentration is established. As the change of absorbance at 617nm is larger
than that of absorbance at 658nm, the 617nm is chosen as the determination wavelength for
Cu (II)-Nitrosulfophenol S-staining protein in the experiment.
Three surfactants were investigated, respectively. The experimental
results indicate that SDS and TritonX-100 do not react with Cu (II)-Nitrosulfophenol S
complex, while TPB make absorbance decrease (shown Fig. 2). Fig.1 and Fig.2 show that the
binding interaction of HSA with Cu (II)-Nitrosulfophenol S complex is similar to the
binding interaction of cation surfactant (TPB) with Cu (II)-Nitrosulfophenol S complex. In
acidic medium at pH2.0, Cu (II)-Nitrosulfophenol S complex have negative charge because of
the ionization of sulfonic acid groups . In the pH range of 1-4, the proteins have
positive charges and molecules present high extension state [7]. Therefore Cu (II)-Nitrosulfophenol S complex reacts with proteins
to form a stable ternary compound (Cu (II)-Nitrosulfophenol S-HSA) by electrostatic
attraction, which is similar to the binding interaction of cation surfactant with the
complex [8,9].
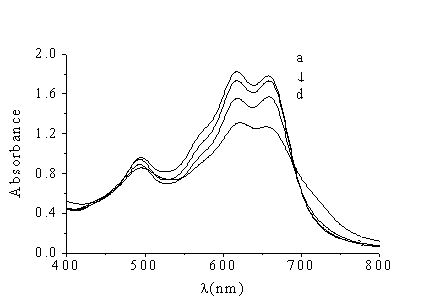
Fig.2 Absorption
spectra of Cu (II)-Nitrosulfophenol S and copper(II)-Nitrosulfophenol S-TPB
a-d. Cu(II) -Nitrosulfophenol S-TPB against water
CTPB (mg/mL): a. 0; b. 10; c. 20; d. 40
3.2 Effect of pH on the reaction
The effect of the different pH values
on the fading reaction was studied. The result implies that DA of the system reaches
a plateau and kept constant in the pH range of 1.8-2.2 and in the dose of buffer solution
range of 1.0-2.0mL. Therefore, 1.5mL buffer solution (pH2.0) was chosen to maintain pH
values of the test solutions.
3.3 Effect of Cu(II) and Nitrosulfophenol S concentration
Cu (II) can react with Nitrosulfophenol S to form 1:1 complex[10]
in acidic medium. In the course of forming complex, it is generally needed to add
excessive ligand or the center ion. When proteins react with Cu (II)-Nitrosulfophenol S
complex, proteins mainly combine with sulfonic acid group of Nitrosulfophenol S by
electrostatic attraction. Therefore, in order to receive stable Cu (II)-Nitrosulfophenol S
complex, the dose of Cu (II) should be increased. The experimental result shows that DA of the system reaches its maximum and
keeps constant when both Cu (II) solution(1.0×10-3mol/L) and Nitrosulfophenol
S(5.0×10-4mol/L) solution are at the range of 0.8-1.2mL. So1.0mL Cu (II)
solution and 1.0mL Nitrosulfophenol S solution were chosen in the experiment.
3.4 Reaction time, temperature and stability of complex
The effect of reaction time was tested at 20°C (room temperature), 25°C and 35°C (water bath), respectively. Results demonstrate that : (1)
the reaction between Cu (II)-Nitrosulfophenol S complex and HSA occurs rapidly at the
above temperatures(<10min).(2) DA is kept constant
within 1 hour. (3) the temperatures have no effect on the reactive system.
3.5 Effect of ion strength and organic solvent
The effect of ion strength on the reactive system was tested with NaCl solution (0.1
mol/L). As Fig. 3 shows, the absorbance difference of the system is constant in the
concentration of NaCl range of 0-0.02 mol/L. The effect of ethanol on the reaction was
studied. When ethanol is in the range of 0-20%(V/V), both the absorbance of Cu (II)-Nitrosulfophenol
S complex (against water) and the absorbance of Cu (II)-Nitrosulfophenol S complex-HSA
(against water) increase. However, the increased degree is not the same, therefore ethanol
has some effect on the system as Fig. 3 shown.
3.6 Effect of surfactants
In order to understand whether surfactants could increase sensitivity of the system,
three surfactants were tried out, respectively. The results are shown in Fig. 3. DA of the system has not been affected
by the neutral TritonX-100. TPB makes DA of the system increased. The reason may be that TPB can react
with Cu (II)-Nitrosulfophenol S complex to form a ternary complex. TPB
participates in the fading reaction, which makes DA of the system increased. On the contrary, SDS makes DA of the system reduced. As SDS (anion
surfactant) are bound to proteins, the surface of the SDS-protein complex [11] will be negatively charged, which greatly affects
the binding interaction proteins with Cu (II)-Nitrosulfophenol S complex because of electrostatic repulsion. At the same
time, it is found that the absorbance of Cu (II)-Nitrosulfophenol S complex(against water) was hardly affected by
SDS. Therefore, DA of the
system reduced.
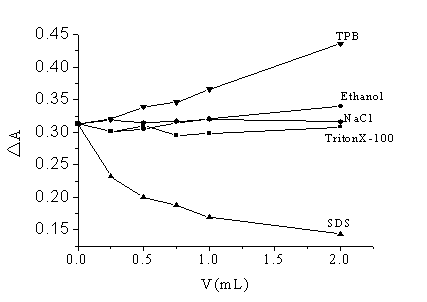
Fig.3 The effect of ion strength, organic solvent and surfactants
(CR=5.0×10-5mol/L ; CBSA=7.35×10-7mol/L
)
1% (V/V) TritonX-100 solution; 100mg/L TPB solution; 100mg/L SDS solution; 0.1 mol/L NaCl
solution
3.7 Precision of the
assay
Ten standard HSA solutions were measured, where each concentration was kept at 7.35×10-7mol/L,
the average absorbance of system was 0.313 at l=617nm, RSD was 2.9%.
3.8 Calibration curves
Calibration curves, linear range, apparent molar absorptivity (e) and Sandell's sensitivity (S) of BSA,
HSA, Hb, g-G,
OVA, Lys were obtained under the optional condition. All the analytical parameters are
listed in Table 1. Obviously, this assay has the following characteristics: wide linear
range, high sensitivity and good response on many proteins.
Table 1 Standard curves for some
proteins
Protein |
Regression equation |
Linear
range |
g |
e |
S |
BSA |
A﹦6.78×10-3c+0.0081 |
0-80 |
0.9998 |
4.61×105 |
0.15 |
HSA |
A﹦7.07×10-3c-0.0409 |
0-80 |
0.9996 |
4.81×105 |
0.14 |
Hb |
A﹦7.05×10-3c-0.0341 |
0-120 |
0.9994 |
4.79×105 |
0.14 |
g-G |
A﹦3.33×10-3c-0.0235 |
0-120 |
0.9995 |
5.99×105 |
0.30 |
OVA |
A﹦4.41×10-3c-0.0391 |
0-60 |
0.9979 |
1.98×105 |
0.23 |
Lys |
A﹦5.13×10-3c+0.0085 |
0-120 |
0.9993 |
0.74×105 |
0.19 |
3.9 Effect of coexisting substances
The influence of coexisting substances, such as amino acids, metal ions, glucose and
citric acid were tested. Results obtained are presented in Table 2. There are twenty basic
amino acids in human body. We tested some typical amino acids, which have more presence in
human serum. Experimental results indicate that none of them have significant interference
on the assay.
Table 2 Effects of Foreign Substances on the determination of 50 mg/L HSA
Substances |
Added |
Error (%) |
Substances |
Added |
Error (%) |
Leu |
40 |
0.53 |
Na+ |
3.22 |
-0.49 |
Ser |
40 |
2.69 |
Al3+ |
0.00017 |
1.48 |
Glu |
40 |
-2.87 |
Fe3+ |
0.001 |
-2.46 |
Lys |
40 |
1.56 |
Mg2+ |
0.025 |
-0.98 |
Cys |
40 |
-3.82 |
Zn2+ |
0.001 |
-4.92 |
Tyr |
40 |
0.53 |
Ca2+ |
0.09 |
0.98 |
Pro |
40 |
-1.52 |
Glucose |
100 |
0.37 |
K+ |
0.168 |
0.49 |
Citric acid |
50 |
1.51 |
3.10 Application on human
serum assay
Based on this method, which has a feature of similar response to many proteins, the
concentration of total proteins of human serum samples was directly determined. Results
are presented in Table 3 and Table 4. Compared with CBB G-250 assay [12], this method has good recovery and reproducibility. There
are disadvantages using CBB G-250 method, the difficulty in preparing of reagents,
instability of reagents, adsorption of reagent to surfaces, and the undesirable
reproducibility. This proposed method will be able to avoid the above disadvantages of CBB
G 250 method.
Table 3 Assay results of total proteins in human serum sample
| Samples | Coomassie
brilliant blue G-250 method |
Cu(II)- Nitrosulfophenol S
method |
1 |
70.5 0.84 |
73.5 0.96 |
2 |
68.7 0.29 |
65.6 1.32 |
3 |
78.9 0.67 |
74.3 0.58 |
Table 4 Recoveries
Samples |
Added/mg |
Total found/mg |
Recovery |
human serum |
- |
0.363 |
- |
0.150 |
0.517 |
102.7% |
|
0.350 |
0.721 |
102.3% |
|
0.550 |
0.902 |
98.0% |
a.mean of three parallel determinations
REFERENCES
[1] Yang R, Liu S. P. Chinese J. Anal. Chem, 2001, 29: 232-236.
[2] Cao Q E, Li Z B, Wang J L.Chinese J. Anal. Chem, 2002, 30: 222-226.
[3] Hu Q H, Liu S P, Luo H Q. Journal of Analytical Scicence, 2002, 18:115-119.
[4 ] Liu D M, Li S T, Zhao S L. Chinese Journal of Analytical Chemistry ,2004, 32:187-190.
[5] Guo Z. X. Shen H X. Analyst, 1999, 124 (7): 1093-1098
[6] Guo Z. X, Hao Y M, Cong X et al. Anal. Chim. Acta, 2000, 403: 225-233
[7] Tanford C, Swanson S A, Shore W S. J. Am. Chem. Soc, 1955, 77: 6414.
[8] Bailey B W, Chester J E, Dagnall R M et al. Talanta ,1968, 15: 1359.
[9] Wei Y J, Li K A, Tong S Y. Chemical Journal of Chinese Universities, 1994, 15
:1470-1472.
[10] Zeng Y E, Zhang H S, Chen Z H. The Content Handbook of Chemical Reagent, fourth ed.
Beijing: Chemical Industry Press, 1993.
[11] Tao W S, Li W, Jiang Y M. Protein Molecule Base. Beijing: Higher Education Press,
1981.
[12] Bradford, M M. Anal. Biochem, 1976, 72: 248-254.
铜(Ⅱ)-硝基磺酚S络合物探针褪色光度法测定蛋白质的研究
胡秋娈a,王春风b,李全民c
(a洛阳师范学院化学系,洛阳,471022; b河南师范大学生命科学学院,新乡,453007;
c河南师范大学化学与环境科学学院,新乡,453007)
摘要 研究发现在pH1.8-2.2的酸性介质中,蛋白质与铜(Ⅱ)-硝基磺酚S络合物发生作用,导致络合物溶液褪色,其最大吸收波长处吸光度的降低值与蛋白质的浓度成正比。以此建立了以铜(Ⅱ)-硝基磺酚S络合物为光谱探针,褪色光度测定蛋白质含量的新方法。该法灵敏度高,线性范围宽,蛋白质(人血清白蛋白)的浓度在0-80mg/ L范围内服从比尔定律,表观摩尔吸光系数e617=4.81×105L·mol-1·cm-1。该体系是三元反应,选择性高,生物体内常见的共存物质均不干扰测定,直接应用于人血清样品中蛋白质总量的测定,结果满意。
关键词 铜(Ⅱ)-硝基磺酚S, 蛋白质, 光谱探针