http://www.chemistrymag.org/cji/2006/084023pe.htm |
Apr. 2, 2006 Vol.8 No.4 P.23 Copyright |
Zhang Yuanming 1, Huang Weiya 1,
Li Hong 2,*, Zhong Mei 3
(1Department of Chemistry, Jinan
University, Guangzhou, 510632; 2 Department of Materials Science and
Engineering, Jinan University, Guangzhou, 510632; 3 Department of Stomatology,
The First Affiliated Hospital of Jinan University, Guangzhou, 510632, China)
Received Jan. 1, 2006.
Abstract The deposition of calcium
phosphate in biomineralization intrinsically depends on the participating of functional
groups in proteins. Mimicking this special growth process in vitro can lead to
further understand the process of biomineralization and synthesize ideal biomaterials for
bone and tooth restoration. In the present study, self-assembled monolayers (SAMs)
terminated with four functional groups (-SO3H, -COOH, -OH and -NH2)
were employed and soaked respectively in two kinds of biomimetic conditions, simulated
body fluid (1.0SBF) at 37oC and 1.5SBF at 50oC for 7 days. XRD, EDS
and SEM results showed that after soaking in 1.0SBF at 37oC for 7 days, a few
calcium phosphate particles were deposited on SAMs with -SO3H, -COOH and -OH,
while not on SAM with -NH2. However, after soaking in 1.5SBF at 50oC
for 7 days, a number of apatite particles were deposited on
all the four kinds of SAMs. The results suggest that -SO3H, -COOH or -OH groups
can induce calcium phosphate deposition by heterogeneous nucleation when biomineralization
progresses in Ca2+ and PO43- supersaturated body fluids.
However, in mineralizing tissue fluids with higher supersaturation degree, all the four
groups can lead to apatite deposition, and among them -NH2 groups may induce
apatite deposition by attracting apatite nuclei from homogeneous nucleation in the
solution.
Keywords Biomineralization; Self-assembled monolayer; Simulated body fluid;
apatite; supersaturation.
In biomineralization, the nucleation and growth of calcium phosphate crystals are modulated by specific proteins in mineralizing tissues, intrinsically by functional groups in proteins. The deposition of crystals within the tissue, their orientation, size and morphology are all carefully regulated [1-3]. The formation of highly mineralized tooth enamel is the exact example of biomineralization in vertebrate tissues. During tooth enamel formation, the concentrations of Ca2+ and PO43- in the tissue fluid were changed at different stages [4,5]. In the secretory stage, calcium phosphate nucleated in the enamel fluid with Ca2+ and PO43- supersaturated. In the following maturation stage, Ca2+ and PO43- ions were extremely increased resulting in a fluid with higher degree of supersaturation and a marked mineralization progressed. The mechanism of tooth enamel formation remains still inconclusive. Therefore, it is necessary to study the functional groups on nucleation and growth of calcium phosphate in different biomimetic solutions.
Biomimetic process is a good way to study the natural process of biomineral and to prepare biologically active materials in vitro, simulating physiological environments of biomineralization. In this process, calcium phosphate can be deposited on substrates by soaking in various Ca2+ and PO43- supersaturated solutions such as simulated body fluid (SBF) which is compositionally similar to human blood plasma, and 1.5SBF in which concentrations of Ca2+ and PO43- ions is 1.5 times than 1.0SBF and it has a higher degree of supersaturation.
Recently, extensive studies have been conducted to investigate functional groups on apatite formation by biomimetic way [6,7]. The functional groups can be successfully introduced onto substrates by using self-assembled monolayer (SAM) technique [8]. Tanahashi et al. found that the ability of inducing apatite deposition in 1.0SBF decreased in the order PO4H2 > COOH >> CONH2 ≈ OH > NH2 >> CH3 [9]. However, Koumoto et al. found that apatite particles could be deposited on SAM terminated with -NH2, but not onto SAM terminated with -OH in 1.5SBF [10]. The ability of apatite deposition on polyamide containing -SO3H has been reported [11], while no relative report to SAM with -SO3H is found to our knowledge. The proteins in tissues like bone or tooth enamel mostly contain functional groups such as -SO3H, -COOH, -OH and -NH2. Therefore, it is necessary to further study the nucleation and growth of apatite on SAMs terminated with -SO3H, -COOH, -OH and -NH2.
In the present study, SAMs with -SO3H, -COOH, -OH and -NH2 terminal groups were prepared. The deposition of calcium phosphate on prepared SAMs were studied by soaking them respectively in two kinds of biomimetic solutions, 1.0SBF at 37 oC and 1.5SBF at 50 oC for 7 days. The possible mechanisms were discussed. 2. EXPERIMENTAL PROCEDURES
2.1 Material and Methods
Si(100) wafers using as substrates were purchased commercially (Guangzhou Research Institute of Semiconductor, Guangzhou, China) and cut into appropriate size (square, 10×10mm). SAMs with -NH2, -OH, -COOH and -SO3H were introduced onto Si(100) substrate as reported (Figure 1) [12,13]. The growth medium, 1.0SBF was prepared according to Kokubo et al. [14] and 1.5SBF was prepared with the concentrations of Ca2+ and PO43- ions 1.5 times larger than those of 1.0SBF. Both 1.0SBF and 1.5SBF were adjusted to pH=7.25 by using appropriate volume of 1M (CH2OH)3CNH2 and HCl. All chemicals used were analytical grade and all of the solutions were prepared with deionized water. The SAMs were floated upside down respectively in vessels containing 100ml 1.0SBF at 37 oC and 1.5SBF at 50 oC for 7 days. After soaking, the SAMs were removed from the solution, rinsed carefully with deionized water and dried at room temperature.
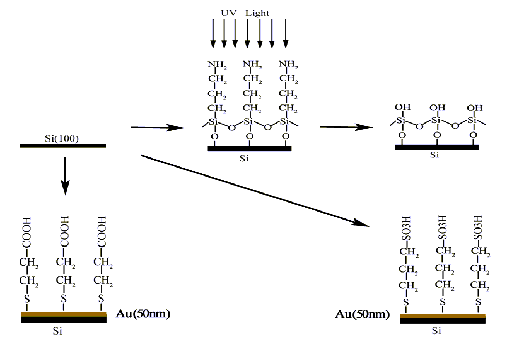
Fig.1 Schematic illustration of the formation of SAMs terminated with -SO3H, -COOH, -OH and -NH2. 2.2 Measurements and Analysis
The static contact angles of water on the prepared SAMs and Si(100) were measured at 25oC using a water-drop contact-angle goniometer (TANTEC Company, CAM-PLUS). Three measurements were made on each sample to get an average value. All the SAMs after soaking in 1.0SBF and 1.5SBF were subjected to XRD (MSAL XD-2) measurements and scanning electron microscope (PHILIPS XL-30 ESEM) observation. All the samples were coated with a thin platinum film before SEM observation. Composition of the particles on SAMs after soaking in 1.0SBF was measured by an energy dispersive spectrometer (EDS, OXFORD ISIT-300).
3. RESULTS AND
DISCUSSION
On cleaned Si(100) substrates, the contact angle was about 50°, while it
increased on SAM with -NH2 because of the hydrophobicity of -NH2
terminal groups, as showed in Figure 2. And the contact angle decreased after exposure to
UV irradiation because the UV light could accelerate the photolytic cleavage of Si-C bonds
and the -NH2 terminal groups decomposed to form -OH terminal groups. The
contact angles of water on SAMs with -COOH and -SO3H were also seen decreased
for the hydrophilicity of -COOH and -SO3H terminal groups. The results
indicated that these functional groups had been successfully fabricated on SAMs, as the
contact angles of water on SAMs strongly depended on the surface functional groups [15].
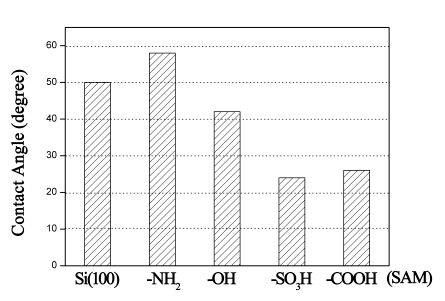
Fig. 2 Contact angles of water on Si(100) and SAMs terminated with
-SO3H, -COOH, -OH and -NH2.
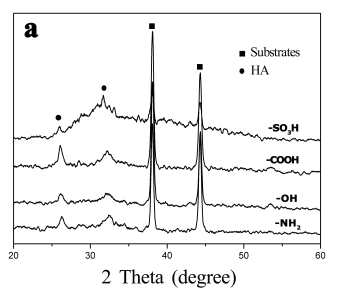
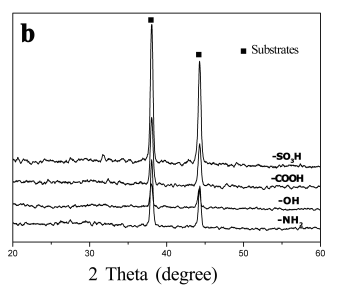
Fig. 3 The XRD patterns of calcium phosphate
crystals on the SAMs terminated with -SO3H, -COOH, -OH and -NH2 (a)
after soaking in 1.5SBF at 50 oC for 7 days (b) after soaking in 1.0SBF at 37 oC
for 7 days.
Figure 3a shows XRD
patterns of the crystals on the surfaces of SAMs after soaking in 1.5SBF at 50 oC
for 7 days. The peaks of 2q=38°
and 44° were ascribed to the diffractions of substrates. All the crystals on the four
SAMs had characteristic apatite peaks with low intensity at 2q=26° and 32°, which meant that apatite with low crystallinity
were deposited. However, no obvious peaks other than the peaks of substrates were detected
on all the SAMs after soaking in 1.0SBF at 37 oC for 7 days (Figure 3b). This
might be because to that the deposited calcium phosphate crystals were too few to be
detected.
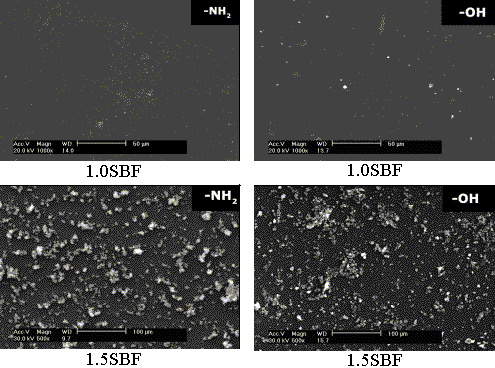
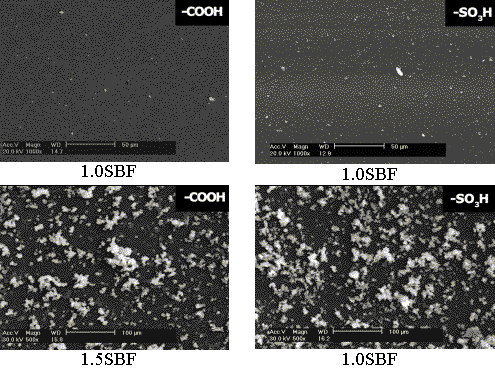
Fig. 4 The SEM images of the SAMs terminated with -SO3H, -COOH, -OH
and -NH2 after soaking in 1.0SBF at 37 oC and in 1.5SBF at 50 oC
for 7 days.
Figure 4 shows the SEM
photos of SAMs terminated with -SO3H, -COOH, -OH and -NH2 functional
groups after soaking in 1.0SBF at 37oC and 1.5SBF at 50oC for 7
days. After soaking in 1.0SBF, a few particles were observed on SAMs with -SO3H,
-COOH and -OH groups, which further confirmed that the deposited calcium phosphate
crystals might be too few to be detected by XRD (EDS results had showed that the deposited
particles had peaks of both Ca and P), while almost no particles could be seen on SAM with
-NH2. However, after soaking in 1.5SBF, a number
of apatite particles were observed on each SAM. There were more particles on SAM with -NH2
than SAM with -OH, and so did SAMs with -COOH and -SO3H. A high magnified SEM
image showed typical apatite morphology on the SAM with -SO3H after soaking in
1.5SBF at 50oC for 7 days (Figure 5). The morphologies of deposited apatite on
the other three SAMs were quite similar to that shown in Figure 5.
The nucleation of apatite can be divided into two types: homogeneous
nucleation occurs in the solution and heterogeneous nucleation occurs on a foreign
substrate. Studies on the mechanisms showed that formation of apatite on the
functionalized surface should be subject to heterogeneous nucleation in SBF [16].
At pH=7.25 in the present experiments, the sulfonic (-SO3H) and carboxyl
(-COOH) functional groups would partially present in their dissociated forms as -SO3-
and -COO-. In addition, -OH could be expected to show
a slight negative charged and -NH2 might become -NH3+ by
capturing proton from the solution [17]. Thus, the negative charged -SO3-
,-COO- and -OH groups could attract Ca2+ ions in the solution via
electrostatic interaction and the accumulation of Ca2+ resulted in a higher
degree of supersaturation near the SAMs/1.0SBF interfaces. Then heterogeneous nucleation
was preferentially triggered on these SAMs. Whereas, the repulsive interaction between NH3+
and Ca2+ ions inhibited heterogeneous nucleation on the SAM with -NH2.

Fig. 5 SEM image of deposited particles on SAM with -SO3H
after soaking in 1.5SBF at 50 oC for 7 days.
In 1.5SBF, however, the
concentrations of Ca2+ and PO43- ions were 1.5 times
larger than those of 1.0SBF. Moreover, the soaking temperature at 50 oC could
result in an even higher super-saturation, because higher temperature decreased the
solubility product of apatite [18]. The increase of super-saturation degree
would initiate the homogeneous nucleation, as we could see some white particles appear in
1.5SBF through naked eyes after soaking for 5 hours. It has been reported that the initial
formed apatite nuclei are negative charged with the formula of [Ca5-x(PO4)3(OH)]2x-
[17]. Therefore, the -NH3+ could attract apatite nuclei
via electrostatic interaction and apatite particles were formed by uptake ions from
solution. The repulsive interaction between -OH groups and apatite nuclei resulted in
relative lesser particles on SAM with -OH. Furthermore, both -SO3-
and -COO- can bind Ca2+ to form complex in the forms of -COOCa+
,(-COO)2Ca and -SO3Ca+, (-SO3)2Ca,
which may act as nucleation sites[11]. The positive charged -COOCa+ and
-SO3Ca+ complex might in turn attract the apatite nuclei from the
solution and accelerate apatite deposition on these two SAMs.
From the above results, it can be deduced that in body fluid condition,
the -SO3H, -COOH and -OH groups in the proteins can regulate the biomineral
process by inducing calcium phosphate deposition, while -NH2 has not the
ability. In the mineralizing tissue fluid with higher degree of supersaturation, all the
four groups can induce calcium phosphate deposition, among which -SO3H, -COOH
and NH2 may play more important roles in apatite deposition during biomineral
process. Further investigations on the functional groups on regulating the orientation,
size and morphology of the calcium phosphate crystals are currently under way in our
research group.
The deposition of calcium phosphate was studied by soaking SAMs with -SO3H, -COOH, -OH and -NH2 terminal groups in 1.0SBF at 37oC and 1.5SBF at 50oC for 7 days. The results showed that in 1.0SBF, calcium phosphate particles were deposited on SAMs with -SO3H, -COOH and -OH groups, while not on SAM with -NH2. However, in 1.5SBF apatite particles were deposited on all the four SAMs. From the results, it can be concluded that when the process of biomineralization progresses in body fluid with Ca2+ and PO43- supersaturated, the -SO3H, -COOH and -OH groups can induce calcium phosphate deposition by heterogeneous nucleation. While in mineralizing tissue fluid with higher degree of supersaturation, all of the four groups can induce apatite deposition, among which -NH2 groups may induce apatite deposition by attracting apatite nuclei from homogeneous nucleation in the solution. Moreover, it can be deduced that the effect on apatite deposition will be changed by adjusting the functional groups in different biomineral conditions. The results are useful for further understanding the process of biomineralization and available for the synthesis of better biomaterials for potential restorative application.
REFERENCES
[1] Iijima M, Moradian-Oldak J, J. Mater. Chem, 2004, 14: 2189-2199.
[2] Fincham A G, Moradian-Oldak J, Diekwisch T G H, et al. J. Struct. Biol, 1995, 115:
50-59.
[3] Toyasawa S, Ohuigin C, Figueroa F, et al. Proc. Natl. Acad. Sci, 1998, 95:
13056-13061.
[4] Takano Y, Crenshaw M A, Reith E J, Calcif. Tissue Int, 1982, 34: 211-213.
[5] Kawamoto T, Shimizu M, Jpn. J. Oral Biol, 1994, 36: 365-382.
[6] Dalas E, Kallitsis K J, Koutsoukos, Langmiur, 1991, 7: 1822-1826.
[7] Bunker B C, Rieke P C, Tarasevich B J, et al. Science, 1994, 264: 48-55.
[8] Bain C D, Evall J, Whitesides G M, et al. J. Am. Chem. Soc, 1989, 111: 7155-7164.
[9] Tanahashi M, Matsuda T, J. Biomed. Mater. Res, 1997, 34: 305-315.
[10] Zhu P X, Masuda Y, Koumoto K, Biomaterials, 2004, 25: 3915-3921.
[11] Kawai T, Ohtsuki C, Kamitakahara M, et al. Biomaterials, 2004, 25(19): 4529-4534.
[12] Zhu P X, Masuda Y, Koumoto K, J. Colloid Interf. Sci, 2001, 243: 31-36.
[13] Zhang J, Kirkham J, Wallwork M L, et al. Langmuir, 1999, 15: 8173-8183.
[14] Kokubo T, Ito S, Huang Z, et al. J. Biomed. Mater. Res, 1990, 24(3): 331-343.
[15] Liu Q, Ding J, Mante F K, et al. Biomaterials, 2002, 23: 3103-3111.
[16] Calvert P, Mann S, Nature, 1997, 386: 127-128.
[17] Zhu P X, Ishikawa M, Seo W S, et al. J. Biomed. Mater. Res, 2002, 59(2): 294-304.
[18] Yamashita K, Oiawa N, Umegaki T, Chem. Mater, 1996, 8: 2697-2700.
张渊明1,黄微雅1,李红2,钟梅3
(1暨南大学化学系,2暨南大学材料科学与工程系,3暨南大学附属第一医院口腔科,广州,510632)
摘要 在生物矿化过程中,钙磷酸盐的沉积本质上取决于蛋白质中官能团的调控作用。体外模拟这一特殊的生长过程有利于进一步了解生物矿化过程和合成理想的骨和牙体修复材料。本文利用自组装技术在仿生环境中研究四种有机官能团的对钙磷盐沉积的作用。将端基为-SO3H、-COOH、-OH 和 -NH2的四种自组装基片分别浸泡于两种仿生环境中: 1.0SBF恒温37℃和1.5SBF恒温50℃生长7天。XRD、SEM和EDS结果表明: 在1.0SBF中恒温37℃浸泡7天后,端基为-SO3H、 -COOH 和 –OH的自组装基片上沉积了少量钙磷酸盐,而在1.5SBF中恒温50℃浸泡7天后,四种自组装基片上都沉积了大量的磷灰石颗粒。本研究表明如果矿化过程在钙、磷离子过饱和的体液中进行,蛋白质中所含的-SO3H、 -COOH 和 -OH基团可以通过异相成核对钙磷酸盐沉积起诱导作用;而在更高过饱和度的矿化组织溶液中,四个基团都能使磷灰石沉积,其中-NH2基团可能通过吸附溶液中均相成核的磷灰石晶核从而诱导磷灰石沉积。
关键词 仿生矿化;自组装基片;模拟体液;磷灰石;过饱和