http://www.chemistrymag.org/cji/2006/088055ne.htm |
Aug. 1, 2006
Vol.8 No.8 P.55 Copyright |
Liu Haiyan 1 Li Shenghui3
Yang Gengliang 1,2
(1 Department of Pharmacy, Hebei University, Baoding 071002, China; 2
Center for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing
100080, China; 3 Department of Chemistry, Hebei University, Baoding 071002,
China)
Abstract Reactive continuous rods
of macroporous poly (glycidyl methacrylate-co-ethylene dimethacrylate) have been prepared
by "in-situ" copolymerization of the
monomers within the confines of a 150¡Á4.6mm I.D. chromatography column in the presence of
porogenic diluent, and b-cyclodextrin
was immobilized on the continuous rods. The effect of the flow rate on the back pressure
drop of the continuous rods was also investigated, which showed that the back pressure was
greatly reduced when comparing to the classical column. By using a pair of nitrophenol
isomeric compounds as the targets, the modified monolithic column was investigated and
good resolution was obtained.
Keywords continuous rods;
1. INTRODUCTION
2. EXPERIMENTAL
2.1 Chemicals
Glycidyl methacrylate(Suzhou Anli Chemicals Co. Ltd.), ethylene dimethacrylate(New Jersey, USA ), azobisisobutyronitrile( The Fourth Factory of Shanghai Reagent), dodecanol, cyclohexanol£¬b-cyclodextrin, (Shanghai Chemical Reagent Company of China Medicine), H2SO4, ammonium dihydrogen phosphate (Factory of Baoding Chemical Reagent).
2.2 Preparation of the monolithic column
The continuous column was prepared by in situ polymerization of monomers within the confines of the stainless-steel tube of a 150¡Á4.6 mm I.D. chromatographic column. First, a mixture consisting of 2.2 mL glycidyl methacrylate, 1.5mL ethylene dimethacrylate(monomers), 0.8 mL dodecanol, 7.0mL cyclohexanol(porogenic diluents), 0.09g azobisisobutyronitrile(initiator) was purged with nitrogen for 10min. Then the stainless steel tube sealed at the bottom was filled with the polymerization mixture and then sealed at the top. After the polymerization was allowed to proceed at 55oC for 24h, the seals were removed from the tube and the column was provided with fittings, attached to the HPLC system and washed with tetrahydrofuran(THF) at a flow-rate of 1mL/min for 60min to remove the alcohols and other soluble compounds present in the polymer rod after the polymerization was completed.
2.3 Preparation of b -cyclodextrin-functionalized porous monolith
The epoxide groups of the poly (glycidyl methacrylate-co-ethylene dimethacrylate) were owned to react with b-cyclodextrin. The amount of the immobilized b-cyclodextrin was determined as described in the literature[18]. Using the optimum conditions, the solution that b-cyclodextrin resolved in the solution of sodium hydroxide was pumped through the column at a flow-rate of 0.1mL/min. The modified columns were then washed with water at a flow-rate of 1mL/min for 2h.
2.4 Chromatography and Instrument
The chromatography system consisted of JASCO (JASCO, Japan) PU-1580 and PU-1586 pumps, a variable wavelength UV-1570 detector. Data processing was carried out with a JASCO LC-1500 workstation. Mobile phases were prepared by mixing methanol and acetonitrile with solution of 50mmol/L ammonium dihydrogen phosphate (7:1:2, v/v/v, pH was adjusted to 4.0 with phosphoric acid). UV3000 spectro-meter (Shimadzu).
3. RESULTS AND DISCUSSION
3.1 Functionalization of the monolithic columns
The amount of
3.2 Separation of aromatic compounds
In order to achieve optimum resolution for m- and o-nitrophenol, the effects of the composition of the mobile phase and pH were investigated. First, we have investigated a series of mixture of methanol and ammonium dihydrogen phosphate solution. When decreased the ratio of methanol, both tR of m- and o-nitrophenol increased, but the maximum resolution was below 1. At the optimum ratio, the effect of pH on the resolution was studied, the results showed that pH 4.0 is better than others, but the resolution still could not exceed 1. So we added acetonitrile into the mixture mobile phase when the ratio of water kept unchanged. The experiments showed that the nitrophenol isomers could obtain better separation on the b -cyclodextrin modified monolithic column when the mobile phase composition was a ternary solvent mixture composed by acetonitrile-methanol-50mmol/L ammonium dihydrogen phosphate solution, pH 4.0(7:1:2,v/v/v). The chromatogram was shown in Figure 1.
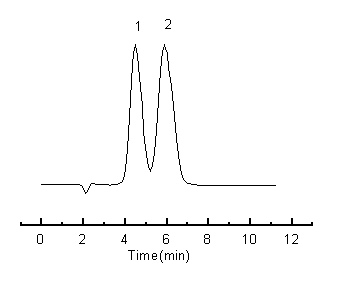
Fig. 1 Separation of nitrophenol isomers on b -cyclodextrin modified monolithic column
1£ºm-nitrophenol£»2£ºo-nitrophenol
4. CONCLUSIONS
The results proved that b -cyclodextrin can be successfully immobilized on the monolithic column and the modified column can be used for the separation of isomers. It means that other chiral ligands are also possible to be immobilized on the monolithic column for separation purpose.
ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of China (Grant No.20375010), "Bai Ren Project" of Chinese Academy of Science and Natural Science Foundation of Hebei University (2005Q25).
REFERENCES
[1] Lee D, J. Chromatogr., 1988,443:143.
[2] Hanson M, Unger K K, LC¨CGC, 1997,15:364.
[3] Regnier F E, Nature, 1991,350:634.
[4] Afeyan N B, Fulton S P, Regnier F E, J. Chromatogr., 1991,544:267.
[5] Wang Q.C., Svec F., Frechet J.M.J., Anal.Chem., 1993,65:2243.
[6] Wang Q.C., Svec F., Frechet J.M.J., J. Chromatogr.A, 1994,669:230.
[7] Wei Y., Huang X., Chen Q. et al. Chin. J. Anal. Chem., 2000,28:1194.
[8] Luo Q., Zou H., Zhang Q. et al. J. Chromatogr.A, 2001,926:255.
[9] Zeng C., Liao J., Nakazato K. et al. J Chromatogr.A, 1996,753:227.
[10]Xie S.F., Svec F., Frechet J.M.J., J. Chromatogr.A, 1997,775:65.
[11] Luo Q., Zou H., Zhang Q. et al. Chin J. Anal. Chem., 2001,29:497.
[12]Liao J., Adv.Chromatogr., 2000, 40:467.
[13] Petro M., Svec F., Frechet J.M.J., Biotech. Bioeng.,1996,49: 355.
[14] Xie S.F., Svec F., Frechet J.M.J., Biotech. Bioeng.,1999,62:30.
[15] Matsui J, Kato T, Takeuchi T et al. Anal. Chem., 1993,65:2223.
[16] Crini G., Morcellet M., Journal of Chromatographic Science, 1996,34:485.
[17] Crini G., Lekchiri Y., Janus L. et al. Chromatogr.,1999,50(11/12): 661.
[18] He B.L., Zhao X.B., Science in China, 1992,12,1240-1247.
¡¡