http://www.chemistrymag.org/cji/2006/ 089056ne.htm |
Sep. 12,
2006 Vol.8 No.9 P.56 Copyright |
Liu Haiyan 1 Li Shenghui3
Yang Gengliang 1,2*
(1 Department of Pharmacy, Hebei University, Baoding 071002; 2
Center for Molecular Science, Institute of Chemistry, Chinese Academy of Science, Beijing
100080; 3 Department of Chemistry, Hebei University, Baoding 071002, China)
Abstract A solid-phase extraction
column packed with diniconazole-imprinted polymers was used successfully to enrich trace
diniconazole. The effects of concentration and flow on the extraction efficiency were
studied. The result showed that the solid-phase extraction column had the ability to
enrich and detect trace diniconazole in aqueous system; the extraction recovery was more
than 93%.
Keywords solid phase extraction; diniconazole; imprinted-polymer;
1. INTRODUCTION
The growing extent of pollution of the environment as a result of human activities
initiated a wide complex of legislative measures. Any assessment of efficiency of
environmental protection policy, however, necessitated availability of relevant and
reliable data on concentrations of pollutants in the environment. The main problems were
encountered in the case of organic micropollutants, where the analysts had to cope with
many different compounds occurring at trace concentrations [1]. Thus, the need
for reliable data on occurrence of organic micropollutants in the environment was an
important driving force initiating the development of modern analytical techniques and
procedures.
In recent years, there has been a growing interest in trace enrichment
techniques that use solid-phase extraction (SPE) as an alternative to the laborious and
time-consuming liquid¨Cliquid extraction (LLE). This
is also due to the high consumption of organic solvents in LLE. SPE has been widely
developed during the last 5 or 6 years with many improvements in format, automation and
also with the introduction of new sorbents with the capability for new ones of trapping
polar analytes[2,3].
Molecularly imprinted polymers (MIPs) are a class of smart materials
with pre-determined selectivity for analytical separation. They hold promise in the
development of highly selective solid phase extraction (SPE) methods for the determination
of trace analytes[4].
Diniconazole is a triazole-type fungicide with a broad antifungal
spectrum. It shows excellent efficacy against various diseases on cereal, fruits and other
field crops by both preventive and curative applications [5]. Because the
widely use of diniconazole, it is necessary to monitor the residues of diniconazole in
drinking water and agriculture products. In France, diniconazole residues in grapes should
not exceed 0.2mg kg-1.
In our previous studies, we have investigated the preparation and the
selectivity of MIPs[6], moreover, we have studied the application of MIPs for
enrichment[7], the extraction efficiency was experimentally determined at
concentrations of 0.1 mg kg-1, 0.2mg kg-1 and 0.5 mg kg-1.
In this work, we have studied the enrichment ability of diniconazole MIP for trace
diniconazole.
2. EXPERIMENTAL
2.1 Reagents and materials
Diniconazole and paclobutrazol were purchased from Factory of Limin (Yancheng, China),
ethylene glycol dimethacrylate(EDMA) from Acros (New Jersey, USA), methacrylic acid(MAA)
from Tianjing Chemical Reagent Company (Tianjing, China), acrylamide(AM) and methanol from
Yili Refined Chemical Co. (Beijing, China), MAA, AM and EDMA were distilled to remove the
inhibitors before the polymerization. 2,2'-azobisisobutyronitrile(AIBN) from Shanghai
Chemical Plant (Shanghai, China) and refined before use. All the other solvents used in
the experiment were HPLC or analytical grade.
2.2 Chromatography
The chromatography system consisted of JASCO
(JASCO, Japan) PU-1587 pumps, a variable wavelength UV-1570 detector; the
flow rate was 1.0 mL min-1. Date processing was carried out with a JASCO
LC-1500 workstation; a water bath was used to retain constant temperatures for
polymerization. UV wavelength was set at 254 nm. An ODS column (JASCO, 100´ 4.6 mm)
and a pre-concentration column (10´ 4.6 mm I.D) packed with MIPs were used.
2.3 Preparation of molecular imprinted
polymers
Diniconazole (1mmol), AM (2mmol), MAA (2mmol) were dissolved in 40mL of toluene in a glass
polymerization test tube, then EDMA (20mmol) and AIBN (50mg) were added into the solution.
The test tube was purged with nitrogen for 10 min and sealed under vacuum. After that,
polymerization was performed in water bath with the temperature maintained at 55¡æ
for 24h. The dried microbeads polymers were packed into the
analytical column and pre-concentration column. Then methanol was used to remove the
template.
2.4 Pre-concentration Sample Preparation
Stock solution was prepared by dissolving 1mg of diniconazole in 1 mL of methanol. The
stock solution was diluted with water to obtain working solutions.
2.5 Solid-Phase Extraction
The pre-concentration column was conditioned with methanol then water and working
solutions were pumped through the pre-concentration column. After sample loading, the pump
system and ODS column were balanced with methanol and water (80: 20, v/v), then the
pre-concentration column was linked to the ODS column and rinsed with the same mobile
phase.
2.6 Extraction Efficiency
The absolute extraction recoveries were evaluated by comparing the analyte peak areas
obtained to those obtained from the corresponding unextracted reference standards prepared
at the same quantity.
3. RESULTS AND DISCUSSIONS
3.1 Effect of concentration
In order to investigate the ability of enrichment and detection of trace diniconazole in
aqueous system, three working solutions were used: 3¡Á10-9mg mL-1, 5¡Á10-9mg
mL-1, 7¡Á10-9mg mL-1. When the flow rate and enrichment
time were kept unchanged, the peak height increased as the concentration of diniconazole
increased. The results showed that the pre-concentration had the ability to detect trace
diniconazole.
3.2 Extraction recovery and the maximum loading capacity
The pre-concentration column had the ability to detect trace diniconazole, but in order to
shorten the enrichment time and quantify accurately, 10-5mg mL-1
working solutions were used in the following experiment. When the flow rate was 1.0mL min-1,
different enrichment times were investigated, the result was shown in Figure 1. From
Figure 1, it could be seen that the peak height increased when enrichment time increased
before the pre-concentration column saturated, moreover, the extraction recovery was also
obtained, the results was shown in Table 1. It could be seen that the extraction
recoveries were more than 93%. After the pre-concentration column reached saturation, the
peak height kept unchanged when enrichment time increased. From Figure 1, the maximum
binding capacity of the pre-concentration column could be obtained and it was 2.4m g.
Under the same condition, no diniconazole was enriched and detected on the blank polymer.
Table 1 Extraction recoveries of diniconazole on MIP column
Enrichment time (h) |
0.5 |
1 |
2 |
3 |
4 |
Theory amount across the column (mg) |
0.3 |
0.6 |
1.2 |
1.8 |
2.4 |
Detected amount (mg) |
0.279 |
0.57 |
1.16 |
1.8 |
2.34 |
Recovery (%) |
93 |
95 |
97 |
100 |
97.5 |
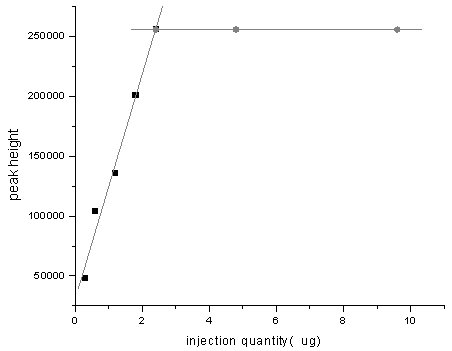
Figure 1 The effect of injection quantity on peak height
3.3 Effect of flow rate
In order to investigate the effect of flow rates on the extraction efficiency, different
enrichment flow rates from 0.5mL min-1 to 1.5 mL min-1 were used.
The results showed that the peak area kept almost invariable when the concentration and
the amount of diniconazole fixed. So the flow rate had no evident influence on extraction
efficiency.
4. CONCLUSION
A solid-phase extraction column packed with diniconazole-imprinted polymers was used
successfully to enrich trace diniconazole. The result showed that the solid-phase
extraction column had the ability to enrich and detect trace diniconazole in aqueous
system, the extraction recovery was more than 93%. So the diniconazole-imprinted polymer
has the potential application in the enrichment, separation and detection of diniconazole
in biological fluids.
ACKNOWLEDGMENTS This work was supported by the National Natural Science Foundation of China (20375010), "Bai Ren Project" of Chinese Academy of Science and Natural Science Foundation of Hebei University (2005Q25).
REFERENCES
[1] Brouwer E.R., Kofman S., Brinkman U.A.Th., Journal of Chromatography A, 1995,703:167.
[2] Eisert R., Levsen K., J. Chromatogr. A, 1996, 733:143.
[3] Font G., Manes J., Molt_o J.C., et al. J.Chromatogr. A, 1993, 642: 135.
[4] J. Olsen, P. Martin, I.D. Wilson, Anal. Commun, 1998,35:13H.
[5] H. Takano, Japan Pestic. Information, 1986,49:18.
[6] H.Y. Liu, G.L. Yang, S.B. Liu, et al. LIQUID CHROMATOGRAPHY & RELATED
TECHNOLOGIES, 2005, 28(15): 2315.
[7] Gengliang Yang, Haiyan Liu, Manman Wang, et al. Reactive and Functional Polymers,2006,
66(5):579-583.