http://www.chemistrymag.org/cji/2006/089057ne.htm |
Sep. 8, 2006
Vol.8 No.9 P.57 Copyright |
(College of Chemistry and Environmental Science, Hebei University; Key Laboratory of Analytical Science and Technology of Hebei Province, Baoding 071002, China)
Abstract The condensation of ketones with
hydroxylamine hydrochloride results ketoximes in good yields in EtOH under ultrasound
irradiation. Compared with conventional methods, the present procedure has several
advantages such as mild conditions, simple reaction procedure, short reaction time and
high yields.
Keywords Condensation; Ketoxime; Hydroxylamine Hydrochloride; Ultrasound
Irradiation
Oximes are highly crystalline and extensively used for the characterization and purification of aldehydes and ketones. These compounds also have other applications such as the preparation of amides,[1] amines,[2] nitric oxide,[3] methylene dioximes[4] and nitriles.[5] Furthermore, oximes have served for the protection of carbonyl groups as exemplified in the synthesis of erythromycin derivatives and perhydrohistrionicotoxin.[6] Oximes may also be used as inhibitors of enzymes. For instances, some oximes are highly active tyrosinase inhibitors for skin lightening or antibrowning, because they may be able to complex the two copper atoms in the active site of tyrosinase;[7] the benzophenone oxime analogues may be used as inhibitor of secretory phospholipase A2 with anti-inflammatory activity;[8] pyridine carbaldoximes and alkyl pyridyl ketoximes act as strong non-competitive inhibitors of the enzymes.[9]
The condensation of primary amine with RR' C=O compound was first reported by Schiff in 1864 and since then a great number of these reactions were performed and reviewed. The experimental conditions depend mostly on the nature of the parent materials and basicity of the reaction medium. In organic chemistry, it is generally believed that reactions of RR'C=O and hydroxylamine at pH close to neutral occurred through nucleophilic attack of the nitrogen electron pair to the electrophilically activated C=O carbon, while in strongly basic media the attacking agent is most likely the anion N HOH or NH2O .[10] Usually, the condensation of carbonyl compounds with hydroxylamine hydrochlorides needs longer reaction time and higher reaction temperature.[7, 11-15] Furthermore, because of the electronic and steric factors, ketones are less reactive to nucleophiles than aldehydes. Therefore, the synthesis of ketoximes is more difficult than the synthesis of aldoximes.
The chemical applications of ultrasound, "Sonochemistry", have become an exciting new field of research during the past decade. Ultrasound has increasingly been used in organic synthesis in recent years. A large number of organic reactions can be carried out in higher yield, shorter reaction time and milder conditions under ultrasonic irradiation.[16] All of those stated above prompted us to study the synthesis of ketoximes in EtOH under ultrasound irradiation (Scheme 1).
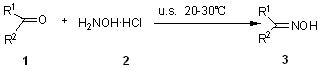
Scheme 1
2 EXPERIMENTAL
Liquid ketones were purified by
distillation prior to use. Melting points were uncorrected. Sonication was performed in
Shanghai Branson-CQX ultrasonic cleaner with a frequency of 25 kHz and a nominal power 250
W. The reaction flasks were located in the maximum energy area in the cleaner (Observation
of the surface of the reaction solution during vertical adjustment of vessel depth will
show the optimum position by the point at which maximum surface disturbance occurs), and the addition or removal of water controlled the temperature of the water bath.
2.1 General procedure
The appropriate ketone (1, 1 mmol) was dissolved in ethanol (5 mL). A solution
of hydroxylamine hydrochloride (2, 1.25 mmol, H2O, 0.5 mL), anhydrous
sodium acetate (1 mmol or 0 mmol) were added. The reaction mixture was irradiated in the
water bath of the ultrasonic cleaner at 25-35 oC for a period as
indicated in Table 1. The mixture was filtered (without filtration, if no NaOAc added).The
solvent was evaporated under reduced pressure. The residue was dissolved in CH2Cl2,
washed with water, and extracted with CH2Cl2. The combined organic
layers were dried over anhydrous MgSO4, filtered, and evaporated to dryness
under reduced pressure. The further purification was accomplished by recrystallization or
by column chromatography on silica (200-300 mesh, eluted with petroleum ether or a mixture
of petroleum ether and diethyl ether). The authenticity of the products was established by
comparing their melting points with the data in the literatures.
Table 1 Condensation of ketones with hydroxylamine hydrochloride under ultrasound irradiation
Entry |
Substrate |
T |
Dehydration agent |
Isolated yield /% |
m.p. / oC (lit.) |
a |
p-ClC6H4COCH3 |
150 |
|
96-98 (97.5-98) [15] |
|
150 |
|
|
|||
300 |
|
|
|||
b |
p-O2NC6H4COCH3 |
150 |
|
174-175 (174) [14] |
|
150 |
|
||||
150 |
|
||||
c |
m-O2NC6H4COCH3 |
180 |
|
129-131 (131-132) [17] |
|
180 |
|
||||
d |
p -CH3OC6H4CHOCH3 |
180 |
|
84-87 (87) [18] |
|
180 |
|
||||
e |
C6H5COC6H5 |
180 |
|
139-142 (141-142) [13] |
|
120 |
|
|
|||
f |
C6H5COCH3 |
120 |
79 |
54-57 (56-58) [19] |
|
90 |
|
|
|||
g |
cyclohexanone |
20 |
|
85-86 (86-88) [20] |
|
20 |
|
|
|||
h |
cyclopentanone |
180 |
|
53-56 (56.5) [21] |
|
180 |
|
|
|||
i |
C6H5CH2COCH2C6H5 |
150 |
|
123-125(125) [21] |
|
90 |
|
|
|||
j |
camphor |
180 |
|
|
113-116 (118) [21] |
k |
anthrone |
180 |
|
no reaction |
b The reaction temp. is 40-45 oC
3 RESULT AND DISCUSSION
The results were summarized in Table 1. It can easily be seen that the condensation of
ketones with hydroxylamine hydrochloride leads to ketoximes in good yields under
ultrasound irradiation. For example, compound 3a was previously prepared in 93.5%
yield in ethanol at reflux temperature for 5 h [15], whereas under
ultrasonication, 3a was obtained in 94% at 20-30oC within 150 min.
We also did experiments in the absence of ultrasound, the condensation
of p-chloroacetophenone and hydroxylamine hydrochloride in the presence of NaOH was
refluxed in ethanol for 5 h to produce p-chloroacetophenone oxime (3a) in
93% yield, the condensation of p-nitroacetophenone and hydroxylamine hydrochloride
was refluxed in ethanol for 150 min to give p-nitroacetophenone oxime (3b)
in 27% yield; 3d was obtained in 13% yield only in refluxed ethanol for 180 min.
(Table 1). It is apparent that the ultrasound can accelerate the condensation reaction of
ketones with hydroxylamine hydrochloride.
The reaction temperature
has little effect on the reaction yield of p-nitroacetophenone oxime (3b).
Under ultrasonication, compound 3b was obtained in 92%
Steric factors also have a role to play in the reactivity of ketones. For example, the acetophenone oxime was prepared in 79% yield in EtOH for 120 min, while the benzophenone oxime was prepared in 17% yield in EtOH for 180 min, and no anthrone oxime was obtained.
4 CONCLUSION
In conclusion, we have found an efficient and convenient procedure for the preparation
of ketoximes via the condensation of ketones with hydroxylamine
hydrochloride under ultrasound irradiation. Compared with the reported, the main
advantages of the present procedure are milder conditions, easier work-up, higher yields
and shorter reaction period.
Acknowledgements The project was supported by Educational Ministry of China, Educational Department of Hebei Province (2001104) and Natural Science Foundation of Hebei Province (B2006000969), China.
REFERENCES
[1] (a) Arisawa M, Yamaguchi M. Org. Lett. 2001, 3: 311;
(b) Luca L D, Giacomelli G, Porcheddu A. J. Org. Chem., 2002, 67: 6272.
[2] Bandgar B P, Nikat S M, Wadgaonkar P P. Synth. Commun., 1995, 25: 863.
[3] Caro A A, Cederbaum A I, Stoyanovsky D A. Biology and Chemistry, 2001, 5: 413.
[4] Shedge A S, Kavitake B P, Desai U V, et al. Synth. Commun., 2004, 34: 4483.
[5] Sarvari M H. Synthesis, 2005, 5: 787.
[6] Shinada T, Yoshihara K. Tetrahedron Lett., 1995, 37: 6701.
[7] Ley J P, Bertram H. J. Bioorg. Med. Chem, 2001, 9: 1879.
[8] Murari S K, Sriharsha S N, Shashikanth S, et al. Bioorg. Med. Chem. Lett., 2004, 14:
2423.
[9] Mlí.ková K, ebela M, Cibulka R, et al. Biochimie., 2001, 83: 995.
[10] Kukushkin V Y, Pombeiro A J L. Coordination Chemistry Reviews, 1999, 181: 147.
[11] Furniss B S, Hannaford A J, Smith P W G, Tatchell A R. Vogel's Textbook of Practical
Organic Chemistry. Longman Harlow, 1989, p. 1259.
[12] Sharghi H, Sarvari M H. Synlett., 2001, 1: 99.
[13] Lachman A. J. Am. Chem. Soc., 1924, 46: 1477.
[14] Cusmano S, Sprio V, Trapani F. Chem. Abs, 1954, 48: 670c.
[15] Campbell K N, Mckenna J F. J. Org. Chem., 1939, 4: 198.
[16] (a) Li J T, Wang S X, Chen G F, et al. Current Organic Synthesis, 2005, 2: 415.
(b) Li J T, Bian Y J, Zang H J, et al. Synth. Commun., 2002, 32: 547.
(c) Li J T, Yang W Z, Wang S X., et al. Ultrason. Sonochem., 2002, 9: 237.
[17] Bodor N, Fey L, Kovendi A. Chem. Abs., 1966, 65: 7011e.
[18] Rogers M A T. Chem. Abs., 1957, 51: 17822d.
[19] Hajipour A R, Mallakpour S E, Imanzadeh G. J. Chem. Research (s), 1999, 228.
[20] Eck J C, Marvel C S. Organic Synthesis 2. 1951, 77.
[21] Cadogan J I G, Ley S V, Pattenden G, Raphael R A, Rees C W. Dictionary of Organic
Compounds. 6rd Ed. London: Cambridge University press, 1996.
李晓亮,李记太
(河北大学化学与环境科学学院,河北省分析科学技术重点实验室,保定 071002)
摘要 超声辐射下,以乙醇为溶剂,酮与盐酸羟胺缩合可得到较好收率的酮肟。与传统的方法相比,该方法有如下优点:温和的反应条件、简单的操作步骤、较短的反应时间和较高的收率。
关键词 超声辐射 酮肟 合成