| http://www.chemistrymag.org/cji/2006/08a064pe.htm | Oct. 10,
2006 Vol.8 No10 P.64 Copyright |
Speciation analysis of trace-level arsenic and selenium in soil using ultrasonic slurry sampling hydride generation-double channel atomic fluorescence spectrometry
Liang Shuxuan1, Lv Tianfeng1, Wen Chunhui1, Sun Hanwen1, Liu Aichang2(1College of Chemistry and Environmental Science, Hebei University, Key Laboratory of Analytical Science and Technology of Heibei Province, Baoding 071002; 2China Metallurgy Perambulation Design Academe of limited company, Bao Ding, 071069, China)
Abstract Ultrasonic aided slurry sampling
hydride generation atomic fluorescence spectrometry (USS–HG-AFS) was developed for the simultaneous determination of total
As, As(III), total Se and Se(IV) in soil samples collected from a sewage-irrigated farm.
500 mg grounded soil was suspended in agar solution by an ultrasound water bath before the
HG-AFS determination. The alternated mixing of the sample solution with thiourea and
ascorbic acid or HCl for the determination of As or Se under optimized conditions was
achieved before the reaction with KBH4. The addition of thiourea and ascorbic
acid promoted the reduction of As (V) to As (III), thus enhancing sensitivity and
precision. The pre-reduction of selenium from Se (VI) to Se (IV) was produced by heating
the sample with HCl. The results for the reference material of soil (serial number
GBW-07411) agreed satisfactorily with the certified values. Results obtained by the
developed procedure compared well with those after traditional acid digestion of samples.
The detection limit are 8.5, 10.5, 3.4 and 6.1 ng L-1 for total As, As(III),
total Se and Se(IV) respectively, with average relative standard deviation values of 3.8,
4.2, 3.1 and 5.6% for analysis of a series of soil samples of different sample sites. The
recoveries of the analytes varied in the range from 95 to 104%. This observation has
stimulated the interest in developing fast, accurate and sensitive analytical methods for
the determination of metal species in soil.
Keywords Ultrasonic slurry sampling; Atomic fluorescence spectrometry; Soil;
Arsenic; Selenium; Speciation analysis
The levels of heavy metals circulating in the environment have been seriously increased during the last decades due to human and industrial activities, but at the same time, it has been growing interest to study the soil, since the soil is a very important abiotic element for all terrestrial ecosystems. It provides the habitat for many invertebrates, which is a source of many nutrients, controls of the circulation of matter in the land environment and the composition of soil inform about the activity of all other phases of land environment. Also the chemical composition of soil is a reliable indication of environmental pollution that quick changes in response to environmental changes[1]. Accumulation of pollutants in soil can result in a high risk for plants and animals, and eventually human health[2]. In this sense, there is an urgent need for analytical methods applied to the determination of trace elements in soil.
The health effects of trace elements are often dependent upon both the quantities and chemical forms of the element. Arsenic and selenium are potential toxic elements that are found in the environment[3,4]. For arsenic, the two most toxic species are arsenite (As(III)) and arsenate (As(V)), respectively, which represent the main forms of arsenic present in soil[5]. In the case of selenium, it is an essential nutrient at low levels of intake and produce toxic symptoms when it is ingested at levels higher than those required for adequate nutrition[6]. The narrow concentration range between the two opposing effects requires accurate and precise knowledge of selenium species present in the environment. In natural soil, selenium exists predominantly in two inorganic forms which are more toxic than organic species[7]. Studies over the years on arsenic and selenium speciation have clearly demonstrated the importance of chemical speciation.
The traditional pretreatment of solid samples to dissolve the metals present is an acid digestion procedure. However, this procedure is time consuming, require adequate laboratory conditions and make use of large quantities of glassware and reagents, increasing the risks of analyte contamination or loss. As the elements exist in different oxidation states, the acid digestion makes them to their highest oxidation states. Therefore, acid digestion is used for the determination of total element content, not for the speciation analysis of elements. An alternative for the pretreatment of solid samples is the slurry formation by using the power and direct analysis of the solid suspension. Compared with traditional sample preparation methods, slurry formation combines the benefits of the solid and liquid sampling, avoiding the contamination and the loss of volatile elements and reduced sample amount needed. Slurry sampling can keep the oxidation states of elements unchanged, so this method is used for the speciation analysis of different elements. This mode has been extensively used in AAS[8-10], ICP-MS[11,12]and AFS[13-15].
Atomic fluorescence spectrometry (AFS) has been used for the determination of hydride-forming elements because of its high sensitivity, wide linear dynamic range, speed of analysis, ease of use, and low cost[16,17]. A literature survey revealed that most of such work concentrated on single element speciation analysis, whereas papers dealing with multielement species determination by hydride generation-double channel AFS are rare.
The main goal of this work is to develop a new analytical method based on the use of ultrasound aided slurry sampling for fast and accurate simultaneous determination of total As, As(III), total Se and Se(IV) in soil by HG-AFS. In this study, the instrument operating parameters, slurry preparation and determination conditions have been optimized. Finally, data for different soil samples were compared with those found after acid digestion, demonstrating that the traditional acid digestion of the samples can be avoided by ultrasonic slurry sampling. This method was applied to the speciation analysis of As and Se in reference material and some soil samples. 2. EXPERIMENTAL
2.1 Instrumentation
A hydride generation-double channel atomic fluorescence spectrometry AFS-230 (Beijing Haiguang Instrumental Co. Ltd., Beijing, China) equipped with hollow cathode lamps(Beijing Vacuum Electronics Research Institute, Beijing, China) were employed for determination of total As, As(III), total Se and Se(IV) throughout this work and the schematic diagram of the system is shown in Fig.1.
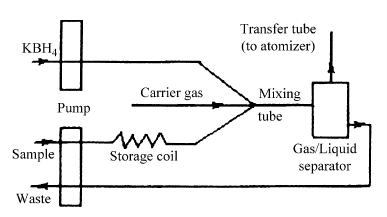
Fig.1 Schematic diagram of the intermittent flow system
The instrumental
parameters and operating conditions were listed in Table 1. The working system can be
programmed in several steps for each measurement, at every step the rotation rate and time
of the two peristaltic pumps used in this work are listed in Table 2.
An ultrasound vibrator (KQ3002B, Kunshan, China) was used to stir the
samples in agar solution before hydride generation. The vessels containing the samples
were placed in a sonicating water bath.
Table 1 Parameters and operating conditions of the HG-AFS instrument
Parameters |
As Se |
PTM voltage (V) |
300 300 |
| Atomizer temperature (oC) | 200 200 |
Atomizer height (mm) |
7 7 |
Lamp current (mA) |
60 80 |
Flow rate of carrier gas (Ar) (mL min-1) |
400 400 |
Flow rate of shield gas (Ar) (mL min-1) |
800 800 |
Read mode |
Peak area |
| Measure method | Std. curve |
Read time(s) / Delay time(s) |
10 / 1 |
Read repeat (times) |
3 |
| Injection volume (mL) | 0.5 |
Table 2 Program of intermittent flow
Step |
Time(s) |
Rotation rate |
Read |
1 |
6 |
0 |
No |
2 |
10 |
100 |
No |
3 |
6 |
0 |
No |
4 |
16 |
120 |
Yes |
2.2 Reagents
The stock solutions of As(III) and Se(IV) (1.0 g L-1) were purchased from
the National Center for Analysis and Testing of Steel Materials(Beijing, China). The
working standards were obtained from the stock solution after dilution with 1%(v/v) HCl
prior to use.
The KBH4 solutions of 20 g·L-1were daily
prepared by dissolving proper amounts of KBH4 (Tianjin Institute of Chemical
Reagents, Tianjin, China) in 0.5% (m/v) NaOH solution.
The thiourea and ascorbic acid solution that was used to reduce As(V)
to As(III) before reaction with KBH4 was prepared by dissolving 10.0 g ascorbic
acid and 10.0 g thiourea in 100mL ultrapure water.
The agar solution (2.0g L-1) was prepared by dissolving 1.0g
agar (Haiyang,Guangdong) in 500mL boiled water.
Aqua regia was prepared by mixing HNO3 and HCl 1:3 (v/v)
from the concentrated solutions.
HCl, NaOH, HNO3,
HClO4and H2SO4 were of high purity grade. All of the
chemicals were of high purity grade available.
2.3 Experimental procedure
2.3.1 Soil properties
The soils used in this study were obtained from the main agricultural areas in Baoding (China) varying widely in physicochemical properties. The soil samples, collected from the surface horizon (0–15cm), were air dried and mildly ground to pass through a 80mm stainless steel sieve, homogenized and stored for subsequent analysis.
2.3.2 Slurries preparation
Two portions of 0.5g soil were weighed accurately in 50mL glass flasks, and 20mL agar solution (2.0g L-1) was added to each one, and shaken energetically. The resulting slurries were dispersed with an ultrasonic vibrator for 15 min in order to obtain homogeneous dispersions.
To one of the portions, which was for total Se and As simultaneous determination, 10 mL conc. HCl and 10mL thiourea-ascorbic acid solution of 10% (m/v) were added, diluted to the mark with ultrapure water and heated in a 95oC water bath for 30 min.
To the second slurry, which was for As(III) and Se(IV) determination, 10 mL conc. HCl was added, diluted to the mark with ultrapure water.
In the two cases 0.5mL of the treated sample were injected into an HCl stream, which is merged with KBH4 to form the hydrides. The analytes were determined in the experimental conditions indicated in Table 1. AFS data found for samples were interpolated in the calibration curve obtained for each element in the same conditions as the samples.
2.3.3 Wet digestion
For comparison, determinations were also performed with wet digestion of soil samples. Test portions (0.5g) were accurately weighed into borosilicate glass vessels and 4mL aqua regia was added. Then the solution was boiling for 2 hours. 2 mL HClO4 was added in the solution, which was going on boiling until dried. The digested samples were transferred to volumetric flasks with 10mL 1%(v/v) HCl.
For total As and Se determination, it is necessary to acidify with 10 mL of conc. HCl and add 10mL of 10% (w/v) thiourea-ascorbic acid solution, diluted to 50 mL with ultrapure water, and then heated at 95oC for 30 min. Digested sample solutions were analysed by HG-AFS following the procedure indicated before for slurries. 3. RESULTS AND DISCUSSIONS
3.1 Pre-reduction conditions
As and Se can exist in two different oxidation states: As(III) and As(V), Se(IV) and Se(VI). In their highest oxidation states, Se(VI) was unable to form hydrides. As(V) was not rapidly deoxidized into its hydride quantitatively. Therefore, pre-reduction of samples is necessary for the determination of total As and Se content.
For deoxidizing Se(VI) to Se(IV), it has been suggested in the literature[18,19] that heated the solution in presence of hydrochloric acid medium which was not lower than 2.0 mol L-1 for about 30 min. To deoxidizing As(V) to As(III), thiourea and ascorbic acid, potassium iodide, hydrochloric hydroxylamine and L-cysteine were commonly used as the prereducing agent. However, there was serious interference to determine Se, it brought about that the signal intensity of Se was usually decreased by a degree of 20–50% than the present method. This aftermath was not acceptable for simultaneous determination of As and Se from a medium except that it was not necessary to pre-deoxidize As(V) to As(III) that could produce much error to determine total As[20]. So Se and As were usually determined from different medium respectively. The investigation was shown that thiourea and ascorbic acid was the much suitable prereducer in the present method. With the presence of it not only As(V)was deoxidized into As(III), but also the signal intensity was not interfered for Se when simultaneous determination of As and Se. 10% (m/v) thiourea and ascorbic acid was employed to all of the determinations.
3.2 Effect of ultrasonic agitation
Ultrasonic agitation has been used as an effective system of homogenizing slurries for HG-AFS and can be used in combination with both manual and automated introduction of the slurry. This sample preparation is more profitable than magnetic agitation and vortex mixing. Hence, in the present work, ultrasonic agitation was used for the slurry preparation.
3.3 Choice of slurry medium
Slurry preparation in aqueous solution is seldom suitable because most powdered materials undergo rapid sedimentation. This sedimentation of suspended material usually occurs after mixing the slurry. The sedimentation rate depends on the densities of the diluent and solid material, the viscosity of the diluent medium and the radius of the sample particles. The slurry can be stabilized using a highly viscous liquid medium. Thus far, agar, glycerol, enthanol and Triton-x100 have been used as slurry stabilizing agents. In this study, the results showed that the viscosity of agar kept different types of particles in suspension for a sufficient time. Furthermore, it has been stated that the optimum concentration of agar was 0.08 %(m/v) for the homogenization of slurry soil samples. Therefore, in the present work, 2.0g L-1 agar solution was used as the slurry stabilizing agent.
3.4 Acidity effects
The generation of nascent hydrogen, which is the actual reducing agent from tetrahydroborate requires an acidic reaction medium. The mechanism of hydride generation that used borohydride as reducing agent under acid conditions was complicated, and the mechanism were related with the characteristics of determined solution and the reagents[21]. HCl, H3PO4, HNO3 and H2SO4 were investigated. As the results of the tests, the signal intensity from hydrochloric acid was higher than those of the other acids.
The signal intensity of of Se(10 mg L-1), As(10 mg L-1) would gradually increased along with increasing hydrochloric acid concentration, and it reached maximal when the concentration of hydrochloric acid was 20% (v/v) for Se and As. The results were presented in Fig.2.
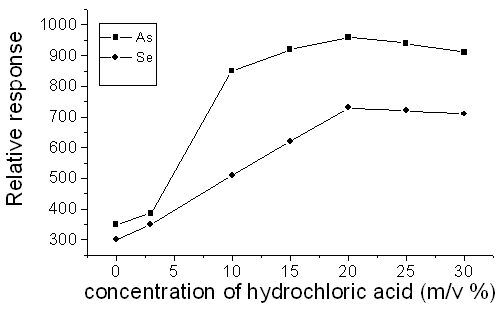
Fig. 2 Effect of the concentration of hydrochloric acid on the atomic fluorescence
3.5 Interference studies
If the determination procedure established is to have any practical utility, it is
essential that it can tolerate the presence of pertinent ions. Thus the influences of
common interferents on the fluorescence signals were investigated. The tolerance content,
defined as the interferent concentration (by wt.) reducing the analyte signal by 10%, are
as follows: 5000-fold K+ and Na+, 4000-fold Ca2+ and Mg2+,
2500-fold Al3+ and V(III), 1000-fold Fe3+, Zn2+ Cu2+,
Mn2+, 500-fold Ag+ and Ni2+, 200-fold Pb2+,
50-fold Bi3+ have no obvious influence on the determination of 20 m
3.6 Analytical performance
The calibration graphs were constructed via determined a set of four element species solutions with concentration equal to 0.0,2.0, 4.0, 6.0, 8.0, 10.0mg·L-1 for each one. Each solution was treated via the same method with the sample solution in step 2.3. Finally, the obtained calibration graph were of very good linearity in linear ranges which were found to be linear up to at least 310mg L-1 for As(III), 320mg L-1 for total As, 35mg L-1 for Se(IV) and 40mg L-1 for total Se, with the correlation coefficient 0.9994,0.9995,0.9992 and 0.9998, respectively.
The detection limits (DL) for four elements species were evaluated on the basis of the standard deviation (S.D.) of the measured signals (11 times) for the blank solution, and were calculated by the formulate DL=3S.D.K-1 (here K is the slopes of the calibration graph). As the results, the detection limits were 8.5ng L-1 for total As, 10.5ng L-1 for As(III), 3.4ng L-1 for total Se and 6.1ng L-1 for Se(IV).
The feasibility of the method was evaluated by analyzing the standard reference material of GBW07411. The results listed in Table 3 reveal that this method is of high accuracy and precision.
Table 3 Analysis of standard reference materials (n=7)
| Soil(GBW07411) | Certified ( mg·g-1) |
Found ( mg·g-1) |
Total As |
205±11 |
200±15 |
Total Se |
0.51±0.13 |
0.48±0.10 |
3.7 Soil sample analysis
The proposed USS-HG-AFS method was applied to the determination of trace amounts of
total As, As(III), total Se and Se(IV) in soil samples collected from suburb cropland. The
total As, As(III), total Se and Se(IV) content of soil samples are shown in Table 4.
Results from USS-HG-AFS are in good agreement with those obtained after acid digestion.
The recovery of total As, As(III), total Se and Se(IV) from soil samples was in the range
of 95-104%. The RSD were 3.8, 4.2, 3.1 and 5.6% respectively. This is indicative of
excellent recovery of those elements.
Table 4 Results from determination of total As, As(III), total Se and Se(IV) in soil samples
Sample* |
total As (mg·g-1) |
As (III) (mg·g-1) |
As3+/total As (%) | total Se (mg·g-1) |
Se (IV) (mg·g-1) |
Se4+/total
Se (%) |
| 1 Slurry Acid digestion |
1.82±0.15 1.85±0.12 |
0.41±0.012 | 22.5 | 0.12±0.016 0.13±0.020 |
0.05±0.002 | 41.7 |
| 2 Slurry Acid digestion |
2.05±0.18 2.12±0.16 |
0.50±0.015 | 24.4 | 0.60±0.035 0.61±0.040 |
0.36±0.010 | 60.00 |
3 Slurry |
1.52±0.11 1.60±0.10 |
0.35±0.010 | 23.0 | 0.55±0.030 |
0.25±0.008 |
45.4 |
4 Slurry |
0.50±0.06 0.52±0.08 |
0.12±0.010 | 24 | 0.32±0.010 |
0.18±0.006 |
56.3 |
5 Slurry |
1.13±0.06 1.15±0.05 |
0.26±0.008 | 23.0 | 0.16±0.015 |
0.08±0.004 |
50.0 |
*
The number of replicate measurements was five. 4. CONCLUSIONThe developed procedure offers an accurate alternative to those based on a previous complete digestion of samples for the determination of total As, As(III), total Se and Se(IV) in soil. The method, based on the excellent sensitivity attainable by AFS and on a soft room-temperature treatment of the samples, is a safe and easy methodology for operators, which reduces the reagent consumption and time of analysis and offers a possibility to explore the presence of total As, As(III), total Se and Se(IV) in soil. The developed method is simple and accurate, so it is promising for speciation analysis of trace of As and Se in other more complex materials.
Acknowledgement This work was supported by Nature Science Foundation of HeBei province, China, for much support to the studied subject.
REFERENCES[1] Han F.X., Banin A., Kingery W.L., et al., Advances in Environmental Research, 2003, 8: 113.
[2] Klavins M., Briede A., Rodinov V., et al., The Science of the Total Environment, 2000, 262: 175.
[3] Bridges C. C., Zalups, Rudolfs K., Toxicology and Applied Pharmacology, 2005, 204: 274.
[4] Savinov V. M., Gabrielsen G. W., Savinova T. N., The Science of the Total Environment, 2003, 306: 133.
[5] Chen H.W., Brindle I.D., Lee X.C., Anal. Chem. 1992, 64: 667.
[6] Roden D.R., Tallman D.E., Anal. Chem. 1982, 54: 307.
[7] Haygarth P.M., Rowland A.P., Sturup S., et al., Analyst.,1993, 118: 1303.
[8] Mierzwa J., Sun Y.C., Chung Y.T., Talanta, 1998, 47: 1263.
[9] Silva D., Erik G.P., Santos, et al., Microchemical Journal,2006, 82: 159.
[10] Mena M.L., Gomez M.M., Palacios M.A., et al, Laboratory Automation and Information Management, 1999, 34: 159.
[11] Wagner B., Garbos S., Bulska E., et al, Spectrochimica. Acta Part B, 1999, 54: 797.
[12] Anderson S. R., Mariana A. V., Adilson J. C., Spectrochimica. Acta Part B, 2004, 59: 243.
[13] Patricia C.M., Cervera M. L., Agust'in P., et al, Talanta, 2004, 62: 175.
[14] Patricia C.M., Eva R.T., ángel M.R., et al, Analytica Chimica Acta, 2004, 506: 145.
[15] Capelo J.L., Fernandez C., Pedras B., et al, Talanta, 2006, 68: 1442.
[16] Greenfield S., TrAC - Trends in Analytical Chemistry, 1995, 14: 435.
[17] Cai Y., Trends in Analytical Chemistry, 2006, 19: 62.
[18] Bryce D.W., Izquierdo A., Castro M.D., Analytica Chimica Acta, 1996, 324: 69.
[19] Bryce D.W., Izquierdo A., Castro M.D., Analytica Chimica Acta, 1995, 308: 96.
[20] Shi J.b., Tang Z.Y., Jin Z.X., et al, Analytica Chimica Acta, 2003, 477: 139.
[21] Liu Z.F., Sun H.W., Shen S.G., et al, Analytica Chimica Acta, 2005, 550: 15.
超声波辅助悬浮进样
-氢化物发生双道原子荧光光谱法测定土壤中的痕量砷和硒及形态分析梁淑轩1 吕天峰1 温春辉1 孙汉文1 刘爱厂2
(1河北大学化学与环境科学学院,河北省分析科学技术重点实验室,071002, 保定;2中勘冶金勘察设计研究院有限责任公司,071069,保定,中国)
摘要 本文采取超声波辅助悬浮进样-氢化物发生原子荧光光谱法同时测定受污水灌溉土壤中的总砷,三价砷和总硒,四价硒含量。取500毫克经过研磨的土壤样品,通过超声波的震荡悬浮于琼脂溶液中。溶液在与硼氢化钾反应前,需要与硫脲-抗坏血酸或者盐酸溶液充分混合。硫脲-抗坏血酸用于将五价砷还原成三价砷,同时增强反应的灵敏度和准确度。六价硒与盐酸溶液混合并加热可以还原成四价硒。通过对标准土壤样品(序列号
GBW-07411)的测定,并测定实际土壤样品中的总砷,三价砷和总硒,四价硒含量并与传统的消化方法进行对比,都证明该方法的准确性。本方法测定总砷,三价砷和总硒,四价硒的检出限可达8.5, 10.5, 3.4 and 6.1 ng L-1,相对标准偏差分别为3.8, 4.2, 3.1 and 5.6%,回收率可达95-104%。该法快捷、简便、具有良好的精密度和准确度,应用于实际样品的测定结果满意。
关键词 超声波辅助悬浮进样,原子荧光光谱法,土壤,砷,硒,形态分析