| http://www.chemistrymag.org/cji/2006/08a066pe.htm | Oct. 10,
2006 Vol.8 No10 P.66 Copyright |
(1 Department of Chemistry, Jinan University; 2 Institute of Nanochemistry, Jinan University, 510632, China)
Received on Sept. 7, 2006; Supported by Foundation of Ministry of Education (No.2002247), Foundation of Jinan University (No.640071) and Foundation of Guangdong province (No.2003C33101).
Abstract Liquid-liquid equilibrium tie line data were determined for the quaternary
system of water + ethanol + diisopropyl ether + n-heptane at 298.15 K and ambient pressure. The experimental liquid-liquid equilibrium results have been successfully correlated by a
modified and an extended UNIQUAC models both with ternary and quaternary parameters in
addition to binary ones.
Keywords Liquid-liquid equilibria, Oxygenated
compounds, Ternary and quaternary mixtures, Modified and extended UNIQUAC models
1. INTRODUCTION
In recent years there has been growing interest in the use of oxygenate additives to
improve gasoline performance as anti-knocking agents and reduction air pollution. Reformulated gasoline includes certain oxygenated compounds such as alcohols and
ethers. These are commonly methanol, ethanol, propanol, and butanol as well as methyl tert-butyl
ether (MTBE), ethyl tert-butyl ether (ETBE), diisopropyl ether (DIPE) and tert-amyl
methyl ether (TAME). DIPE is an ideal fuel additive to replace MTBE because of its high
octane number and lower vapor pressure. To assess the solubility of DIPE in the alcohol
aqueous with hydrocarbon, we continue to study the phase behavior of liquid alcohol
aqueous mixtures of DIPE with gasoline-substitution hydrocarbons. Here we report liquid-liquid equilibrium (LLE) measurements on one quaternary system
water + ethanol + DIPE + n-heptane at 298.15 K. The experimental LLE data were correlated by
means of the modified UNIQUAC and extended UNIQUAC models [1,2] including both ternary and quaternary parameters coming from multicomponent
intermolecular interactions, in addition to binary parameters. The constituent ternary
systems of water + ethanol + n-heptane, water + ethanol + DIPE [3],
water + DIPE + n-heptane [4], were used to obtain
ternary parameters for accurate representation of the quaternary LLE system studied in
this work. The binary parameters of miscible binary mixtures of constituents of the
ternary and quaternary systems were obtained from vapor-liquid equilibrium data [5-8] and those of immiscible mixtures were obtained from mutual solubility
data [3,9].
2. EXPERIMENTS
2.1 Materials
DIPE was provided by the Tianjin Kermel Chemical Reagents Development Center, with a
mass fraction of 99.5 % at the fewest. Ethanol was supplied by Guangzhou Chemical Reagent
Factory, with a minimum mass fraction of 99.5 %. n-Heptane was supplied by the
Guangzhou Chemical Reagent Factory, with a minimum mass fractions of 99.7 %. All chemicals
were used without further purification. For GC analysis, appreciable peaks have not been
detected. Water was distilled twice.
2.2 Apparatus and Procedures
Ternary and quaternary LLE measurements were carried out at 298.15 ± 0.01 K. The
quaternary mixtures were prepared by mixing stepwise the binary DIPE + n-heptane
mixtures whose compositions are M1, M2, and M3
with water, and then ethanol to cover the two-phase regions. The values of M1,
M2, and M3 are approximate 0.25, 0.50, and 0.75,
respectively, indicating the mole fraction of DIPE in the binary DIPE + n-heptane
mixtures. About 70 cm3 of each mixture was poured into the equilibrium glass
cell placed in a thermostated water bath. The mixture was then stirred vigorously by
magnetic stirrer for 3 h and then allowed to settle for 3 h, which was sufficient to
separate into two liquid phases. Dry nitrogen gas was used to prevent contamination from
moisture in the headspace of the equilibrium cell. Samples, withdrawn from upper and lower
phases in the cell by a microsyringe, were analyzed by a gas chromatograph (GC-14C)
equipped with a thermal conductivity detector. Each component of the ternary and
quaternary mixtures was separated clearly, using a stainless steel column (2 m long, 3 mm
i.d.) packed with Porapak QS. The temperatures of the injection system and detector system
were all set at 513.15 K. The initial
temperature and final temperature of the oven was kept at 483.15 K and 453.15 K,
respectively. The hydrogen flow rates for both the separation and reference columns were
set at 1.1 cm3 s―1. The peak areas of the components, detected with a
chromatopac (N2000), were calibrated with prepared known mixtures by mass. The mass of
each component of the mixture was determined from the calibration and converted to mole
fraction. Three analyses were done for each sample to obtain a mean value with a
reproducibility of better than 0.1%. The accuracy of the measurements was estimated within
±0.001 in mole fraction.
2.3 Experimental results
Tables 1 and 2 show experimental LLE
data for the water + ethanol + n-heptane and
water + ethanol + DIPE + n-heptane mixtures.
Table 1 Equilibrium phase compositions in mole fraction for the ternary mixtures of water (1) + ethanol (2) + n-heptane (3) at 298.15 K
Organic phase |
Aqueous phase |
||||
|
|
|
|
|
|
0.0007 |
0.0000 |
0.9993 |
1.0000 |
0.0000 |
0.0000 |
0.0020 |
0.0014 |
0.9966 |
0.9675 |
0.0325 |
0.0000 |
0.0025 |
0.0047 |
0.9928 |
0.8969 |
0.1031 |
0.0000 |
0.0024 |
0.0119 |
0.9857 |
0.7890 |
0.2110 |
0.0000 |
0.0026 |
0.0151 |
0.9823 |
0.7440 |
0.2560 |
0.0000 |
0.0030 |
0.0209 |
0.9761 |
0.6731 |
0.3269 |
0.0000 |
0.0043 |
0.0263 |
0.9694 |
0.6098 |
0.3882 |
0.0020 |
0.0040 |
0.0311 |
0.9649 |
0.5595 |
0.4371 |
0.0034 |
0.0050 |
0.0372 |
0.9578 |
0.5049 |
0.4878 |
0.0073 |
0.0053 |
0.0449 |
0.9498 |
0.4525 |
0.5362 |
0.0113 |
0.0080 |
0.0556 |
0.9364 |
0.3967 |
0.5860 |
0.0173 |
0.0075 |
0.0775 |
0.9150 |
0.3153 |
0.6499 |
0.0348 |
0.0141 |
0.1244 |
0.8615 |
0.2531 |
0.6779 |
0.0690 |
Table 2 Equilibrium phase compositions in mole fraction for the quaternary mixtures of water (1) + ethanol (2) + DIPE (3) + n-heptane (4) at 298.15 K
Organic phase |
Aqueous phase |
||||
|
|
|
|
|
|
{ |
|||||
0.0233 |
0.0116 |
0.2339 |
0.9485 |
0.0507 |
0.0008 |
0.0379 |
0.0495 |
0.2218 |
0.8636 |
0.1352 |
0.0012 |
0.0382 |
0.0613 |
0.2168 |
0.8277 |
0.1708 |
0.0015 |
0.0571 |
0.0889 |
0.1937 |
0.7916 |
0.2058 |
0.0026 |
0.0623 |
0.1077 |
0.1760 |
0.7673 |
0.2289 |
0.0038 |
0.0709 |
0.1407 |
0.1615 |
0.7227 |
0.2692 |
0.0053 |
0.0839 |
0.2225 |
0.1349 |
0.7033 |
0.2873 |
0.0064 |
0.0982 |
0.2760 |
0.1203 |
0.6621 |
0.3270 |
0.0077 |
0.0081 |
0.0088 |
0.4625 |
0.9766 |
0.0226 |
0.0008 |
0.0169 |
0.0210 |
0.4555 |
0.9408 |
0.0581 |
0.0011 |
0.0278 |
0.0363 |
0.4457 |
0.9082 |
0.0904 |
0.0014 |
0.0500 |
0.0693 |
0.4105 |
0.8634 |
0.1344 |
0.0022 |
0.0574 |
0.0941 |
0.3908 |
0.8318 |
0.1643 |
0.0039 |
0.1026 |
0.2250 |
0.3205 |
0.8074 |
0.1874 |
0.0052 |
0.1289 |
0.2656 |
0.2844 |
0.7641 |
0.2266 |
0.0084 |
0.1880 |
0.3062 |
0.2487 |
0.7345 |
0.2515 |
0.0120 |
0.2004 |
0.3329 |
0.2295 |
0.7016 |
0.2830 |
0.0141 |
0.0409 |
0.0168 |
0.6934 |
0.9777 |
0.0216 |
0.0007 |
0.0496 |
0.0358 |
0.6767 |
0.9622 |
0.0367 |
0.0011 |
0.0569 |
0.0839 |
0.6362 |
0.9075 |
0.0909 |
0.0016 |
0.0847 |
0.1373 |
0.5661 |
0.8728 |
0.1239 |
0.0033 |
0.0928 |
0.1670 |
0.5438 |
0.8301 |
0.1646 |
0.0053 |
0.1116 |
0.2431 |
0.4637 |
0.8034 |
0.1881 |
0.0085 |
0.1396 |
0.2724 |
0.4174 |
0.7706 |
0.2176 |
0.0118 |
0.1513 |
0.3028 |
0.3901 |
0.7265 |
0.2545 |
0.0179 |
3. CALCULATION PROCEDURE AND RESULTS
3.1 Calculation procedure
We have used the modified UNIQUAC[1] and extended UNIQUAC[2] models with binary and additional ternary and quaternary
parameters for an accurate description of the experimental quaternary LLE data and
constituent ternary data as well as binary VLE and mutual solubility data.
The binary parameter ![]() defined by the binary energy parameter aji is expressed as
defined by the binary energy parameter aji is expressed as
![]() (1)
(1)
where aji can be obtained from binary experimental
phase equilibrium data, and C was set to 1 for the extended UNIQUAC and 0.65 for
the modified UNIQUAC.
The binary energy parameters for the miscible mixtures were obtained
from the VLE data reduction using the following thermodynamic equations[10]:
![]() (2)
(2)
![]() (3)
(3)
![]() (4)
(4)
![]() and
and ![]() ( I, II = two
liquid phases ) (5)
( I, II = two
liquid phases ) (5)
The ternary and quaternary LLE calculations were carried out using the Eqns.(4) and (5). For the ternary systems of type 1 having a plait point, two-parameter UNIQUAC models predict generally larger solubility envelope than the experimental one. It is necessary to correlate ternary and quaternary LLE using ternary and quaternary parameters in addition to binary ones. The additional ternary parameter
tijk was obtained by fitting the model to the ternary experimental LLE data and the quaternary parameter tijkl was determined from the quaternary experimental LLE data using a simplex method[15] by minimizing the objective function: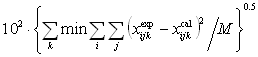 (6)
(6)where min means minimum values, i = 1 to 3 for ternary mixtures or i =1 to 4 for quaternary mixtures, j = phases I and II, k = 1,2,…,n (no. of tie lines), M = 2ni, and x = (the liquid-phase mole fraction).
3.2 Calculation results
Table 3 presents the constituent binary energy parameters of the modified and extended UNIQUAC models. Table 4 shows the ternary parameters obtained in fitting the modified and extended UNIQUAC models to the experimental ternary LLE systems, and root-mean-square deviation of the mole fraction of tie lines between the experimental and calculated results for the ternary LLE systems. It seems that the modified UNIQUAC model with the only binary parameters predicts the ternary LLEs more successfully than the extended UNIQUAC model, and these models can give a much more accurate representation for the ternary LLEs by including the ternary parameters in addition to the binary ones. Figure 1 compares the experimental and correlated liquid-liquid equilibrium results of three boundary ternary systems making up the quaternary system at T = 298.15 K. It shows good agreement between the experimental values and those correlated using additional ternary parameters in Figure 1. The quaternary system exhibits type 2 quaternary liquid-liquid behavior[16], which are composed of two ternary liquid-liquid equilibrium for the mixtures (water + ethanol + DIPE) and (water + ethanol + n-heptane) classified as type 1, and one ternary liquid-liquid equilibrium for the mixtures (water + DIPE + n-heptane) as type 2.
Table 4 summarizes the quaternary LLE results predicted by the modified UNIQUAC and extended UNIQUAC models with the binary and ternary parameters, together with root-mean-square deviations. The root-mean-square (r.m.s.) deviations predicted using the binary and ternary parameters are slightly large for the water + ethanol + DIPE + n-heptane system, but both models can describe accurately the quaternary experimental LLE data by the correlation involving the additional quaternary parameters, and the correlated r.m.s. is 1.88 mol% for the modified UNIQUAC model and 4.13 mol% for the extended UNIQUAC model.
Table 3 Calculated results of binary phase equilibrium data reduction
System (1+2) |
T /K |
No. of data points |
Model |
Energy parameters |
Ref. |
|
a12/K |
a21/K |
|||||
ethanol + water |
298.15 |
10 |
Ia |
212.17 157.12 |
-46.98 |
[5] |
ethanol + n-heptane |
298.15 |
19 |
I |
107.23 |
1327.88 |
[6] |
ethanol + DIPE |
344.17 |
9 |
I |
-24.30 |
701.41 |
[7] |
DIPE + n-heptane |
342.35~363.45 |
10 |
I |
244.92 |
-168.50 |
[8] |
DIPE + water |
298.15 |
MSc |
I |
1590.60 |
166.68 |
[3] |
n-heptane + water |
298.15 |
MS |
I |
1884.20 |
1022.10 |
[9] |
a
Modified UNIQUAC model;b Extended UNIQUAC model;
c Mutual solubility.
Table 4 Calculated results for ternary liquid-liquid equilibrium at 298.15 K
ternary parameters |
deviations f |
|||||||
system (1+2+3) |
no.a |
model |
t231 | t132 | t123 | predd |
corre |
lit. |
| water + ethanol + n-heptane | 13 |
Ib |
-0.2730 |
-0.9130 |
0.2883 |
4.78 |
0.55 |
this work |
IIc |
-0.6339 |
0.0300 |
-0.3743 |
13.61 |
0.65 |
|||
water + ethanol + DIPE |
8 |
I |
-0.4224 |
1.4848 |
-1.7460 |
1.78 |
1.42 |
[3] |
II |
-0.4125 |
0.5337 |
-3.1172 |
4.23 |
1.57 |
|||
| water + DIPE + n-heptane | 11 |
I |
0.0270 |
0.3098 |
0.2238 |
0.51 |
0.22 |
[4] |
II |
0.0158 |
-0.1784 |
-0.3343 |
0.47 |
0.44 |
|||
a
Number of tie lines.b Modified UNIQUAC model
c Extended UNIQUAC model.
d Predicted results using only binary parameters.
e Correlated results using binary and ternary parameters.
f Root-mean-square deviation (mol%).
Table 5. Calculated Results for Quaternary Liquid-Liquid Equilibria at 298.15 K
system |
quaternary parameter |
deviation f |
||||||
(1+2+3+4) |
no.a |
model |
t2341 | t1342 | t1243 | t1234 | predd |
corre |
water + |
24 |
Ib |
-0.8303 |
15.8975 |
31.1354 |
-19.6513 |
4.04 |
1.88 |
IIc |
-0.0105 |
-0.0012 |
0.0840 |
-2.7774 |
7.61 |
4.13 |
||
a
Number of data points.b Modified UNIQUAC model.
c Extended UNIQUAC model.
d Predicted results using binary parameters alone.
e Correlated results using binary and quaternary parameters.
f Root-mean-square deviation(mol%).
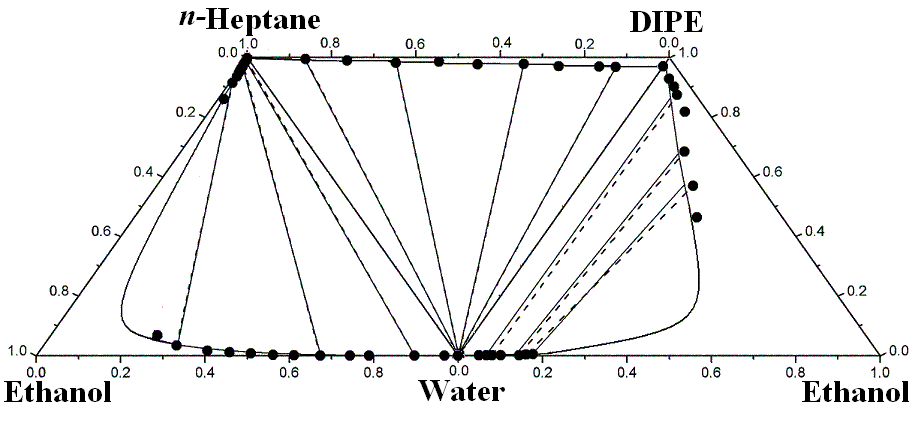
Figure 1 Experimental and calculated (liquid + liquid) equilibria of three ternary mixtures making up of (water + ethanol + DIPE + n-heptane) at T = 298.15 K. ●- - -●, Experimental tie line; ——, correlated by the modified UNIQUAC model with binary and ternary parameters taken from Tables 3 and 4.
4. CONCLUSION
The quaternary LLE of the water + ethanol
+ DIPE + n-heptane system were
measured at 298.15 K in this work. The experimental quaternary liquid-liquid equilibrium data were successfully correlated by using both
models including binary, ternary and quaternary parameters. The quaternary liquid-liquid equilibrium results calculated by the modified UNIQUAC model
are more suitable agreement with the experimental results.
REFERENCES
[1] Tamura K, Chen Y, Yamada T et al. J
Solution Chem, 2000, 29(5): 463-488.
[2] Nagata I. Fluid Phase Equilibria, 1990, 54: 191-206.
[3] Arce A, Marchiaro A, Rodriguez O et al. J
Chem Eng Data, 2002, 47: 529-532.
[4] Chen Y, Dong Y. J Solution Chem, 2005, 34(12):
1445-1457.
[5] Hall D J, Mash C J, Penberton R C. NPL Report Chem, 1979, 95: 1-32.
[6] Hongo M, Tsuji T, Fukuchi K et al. J Chem Eng Data, 1994, 39: 688-691.
[7] Lee M-J, Hu C-H.
Fluid Phase Equilibria, 1995, 109: 83-98.
[8] Vijayaraghavan S V, Deshpande P K, Kuloor N R. J Chem Eng Data, 1967, 12: 15-16.
[9] Sørensen J M, Arlt W. DECHEMA Chemistry Data Series, Vol. V, Part 1; DECHEMA:
Frankfurtam am Main, Germany, 1979.
[10] Prausnitz J M, Anderson T F, Grens E A, et al. Computer Calculation for
Multicomponent Vapor-Liquid and Liquid-Liquid Equilibria . Prentice-Hall: Englewood
Cliffs, NJ, 1980:19.
[11] Riddick J A, Bunger W B, Sakano T K. Organic Solvents; 4th ed.; Wiley-Interscience:
New York, 1986.
[12] Reddy S K V N, Prasad H L D, Krishnaiah A. J Chem Eng Data, 2004, 49: 1546-1549.
[13] Spencer C F, Danner R P. J Chem Eng Data, 1972, 17: 236-241.
[14] Hayden J G, O'Connell J P. J Ind Eng Chem Process Des Dev, 1975, 14: 209-216.
[15] Nelder J A, Mead R. J Computer, 1965, 7: 308-313.
[16] Sørensen J M, Arlt W. Liquid-Liquid
Equilibrium Data Collection, Ternary Systems; DECHEMA Chemistry Data Series; Vol. V, Part
2; DECHEMA: Frankfurt am Main, Germany, 1980.
陈瑶1,2 董艳辉1,2, 张胜利1,2
(1 暨南大学化学系;2 暨南大学纳米化学研究所,广州,510632)
摘要 测定了水、乙醇、二异丙醚和正庚烷四元体系以及相关的三元体系水、乙醇和正庚烷在298.15K和常压下的液液相平衡数据,含有二元、三元和四元参数的modified UNIQUAC 和 extended UNIQUAC 热力学模型成功地关联了这些实验数据。
关键词 液液平衡,含氧化合物,三元和四元混合物,Modified和extended UNIQUAC 热力学模型