| http://www.chemistrymag.org/cji/2006/08b068pe.htm | Nov. 1,
2006 Vol.8 No11 P.68 Copyright |
(College of Chemistry and Environmental Science, Hebei University, Baoding, 071002) Received Oct. 11, 2006.
Abstract An investigation was
undertaken regarding the adsorption behaviors of copper (II) and lead (II) ions from
aqueous solutions by crosslinked starch graft copolymers with aminoethyl groups, which
were synthesized by grafting 2-aminoethyl methacrylate onto crosslinked starch. The
dynamic adsorption method was utilized to evaluate adsorbability of graft copolymer under
various parameters such as metal ion concentration, adsorption time, grafting percentage
and adsorption temperature. The adsorption time reaching equilibrium for Cu (II) and Pb
(II) was found to be two hours and one hour, respectively. The adsorption capacity
increases with the increasing of graft percentage and metal ions concentration. For the
starch graft copolymer with grafting percentage of 75.5%, when the metal ions
concentration was about 5.0 mmol/L, the saturation adsorption capacity of Cu (II) and Pb
(II) are 21.05 mg/g and 144.08 mg/g (dry weight), respectively, and its desorption
percentage is about 95%.
Keywords : Adsorption; Heavy metal ions; Starch graft copolymer;
Wastewater treatment
1 INTRODUCTION
Many efforts have been focused on the removal of some heavy metal ions such as Pb
(II), Cu (II), Hg (II), Ag (I) and Cr (VI) from industrial effluents, mine water and water
supplies due to their toxicity to human and natural wildlife in recent years [1-5].
It is well known that heavy metal ions released into the environment are highly toxic to
the living organisms and change ecological balance by environmental cycling [6].
Consequently, various methods including reverse osmosis, ion-exchange, adsorption and
electrodialysis techniques have been developed in order to remove or recover the heavy
metal ions from all kinds of waste water [7-8]. Among the various separation
and pre-concentration techniques, adsorption of heavy metal ions on many water-insoluble
stationary phases has been widely investigated. Some functionalized polymers, silica and
activated carbon with chelating groups have become the most favored adsorbents due to
their good mechanical strength, large surface, fast adsorption, inexpensiveness and
availability [9-10]. Recently, the adsorbents based on natural products and
their derivatives deserved particular attentions because of an increasing interest in the
removal of heavy metal ions from waste water. For example, crosslinked amphoteric starch
with quaternary ammonium groups can effectively remove Cr (VI), Cu (II) and Pb (II) ions
in aqueous solution [11-13]. Zhang et al reported the adsorption
behaviors of Cu (II) onto water-insoluble phosphate carbamate and its maximum adsorption
capacity was 1.60mmol/g [14]. Also, chitin, its derivatives [15] and
modified cellulose [16] have been studied with respect to their ability to
remove heavy metals from aqueous solution.
Starch is one of the important agricultural biopolymers used in the
industry due to its low cost, renewability and biodegradability. However, starch by itself
can not be directly applied in adsorbing heavy metal ions because it has inherently no
chelating or complexing abilities. Therefore, it is very necessary that functional groups
are introduced into the starch backbone by grafting method to obtain the effective
adsorbents for heavy metal ions. Dong et al reported the adsorption behaviors of
Cu(II) onto cornstarch copolymer with dimethylaminoethyl methacrylate and gave the optimal
adsorption condition [17].
In this study, the newly-synthesized starch graft copolymers containing
aminoethyl groups was used for removing Cu (II) and Pb (II) ions in aqueous solution by
the effective complexation of amine group with Cu (II) and Pb (II) ions. The effects of
various parameters such as metal ions concentration, adsorption time, adsorption
temperature and grafting percentage of the starch graft copolymers were investigated.
Moreover, the adsorbed Cu (II) and Pb (II) ions can be easily desorbed by treating with
HCl solution and the desorption percentage reached above 95% when desorbing with 1 N HCl
solution for 1 h at room temperature.
2.1 Materials
Commercial crosslinked starch powder, from Guangxi Starch Factory in China, was dried at 105oC before use. 2-aminoethyl methacrylate hydrochloride (AEMH) was purchased from Acros, 99%. All solutions of copper (II) sulfate and plumbum (II) acetic were prepared using deionized water. The other reagents and solvents were commercially available and were used without further purification.
2. 2 Synthesis of starch graft copolymer
Four starch graft copolymers with different grafting percentages (GP) (27.5%, 50.0%, 75.5% and 100.3%), used as the absorbents for Cu (II) and Pb (II) ions in this work, were prepared by varying the monomer concentration. In a typical graft copolymerization, 2 g of crosslinked starch and 50 mL deionized water were added into a 100 mL three-neck flask equipped with a stirrer and immersed in a thermostat water bath. The N2 gas was purged into the flask to remove the oxygen during the reaction. The required amount of AEMH was added into the flask at 45oC in order to keep acidic reaction system. Then, a conventional grafting initiator for starch, Ce+4 ion (2.0×10-3 mol/L) was injected into the flask with syringe to initiate the graft copolymerization of AEMH onto starch. After a given time, the stoichiometric NaOH aqueous solution was added into the flask in order to convert AEMH into 2-aminoethyl methacrylate moiety. Then the reaction mixture was added to cool water to remove the unreacted monomer and the possibly formed homopolymer of 2-aminoethyl methacrylate. The crude graft starch copolymer was filtered and extracted by Soxhlet extraction with acetone for 24h. The final starch graft copolymer was then dried to a constant weight under vacuum at 60oC for 24 h, weighed and calculated the grafting percentage. The grafting percentage was calculated according to the following equation:
Grafting percentage % (GP) = (Polymer in graft/Weight of substrate) × 100
2. 3 Adsorption procedure
The adsorption experiments were carried out in a series of flasks containing the desired dose of the grinded starch graft copolymer and 20 mL Cu (II) or Pb (II) solution at the desired concentration in a constant temperature bath. After stirring for a fixed adsorption time, the adsorbents were separated from the adsorption media and the concentration of Cu (II) or Pb (II) ions after adsorption was determined by the chemical titrimetric analysis. The adsorption capacity was calculated from the following equation:
Q = (C0-C1) V﹡M / m
where Q is the adsorption capacity of starch graft copolymer (mg/g); C0 and C1 (mol/L) are the initial and final concentrations of Cu (II) and Pb (II) in the adsorption medium, respectively, V (mL) and m (g) are the volume of Cu (II) solution and the amount of the starch graft copolymer, respectively, and M is the molecular weight of Cu (II) or Pb (II).
3 RESULTS AND DISCUSSION
3.1 Structure-conforming of starch graft copolymer
As shown in Fig. 1, the grafting copolymer was conformed by comparing the IR spectrum of
pure starch with its graft copolymer. The main difference observed is the appearance of an
absorption band at 1732 cm-1 which is ascribed to the carbonyl group of
2-aminoethyl methacrylate in the IR spectrum of starch graft copolymer. Also, the
characteristic absorption at 1575 cm-1 can be attributed to the deforming
absorption of N-H bond, which were not observed in the IR spectrum of the pure starch. The
above-mentioned results clearly indicated that the monomers 2-aminoethyl methacrylate have
been successfully grafted onto the macromolecular chains of starch.
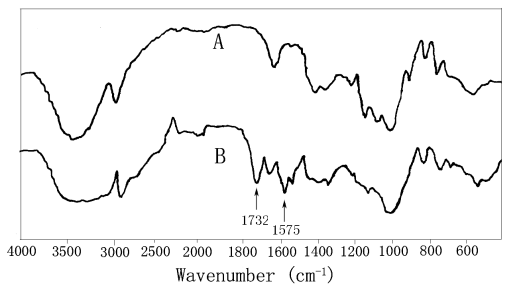
Fig.1 IR spectra of pure starch (A) and
starch graft copolymer (B)
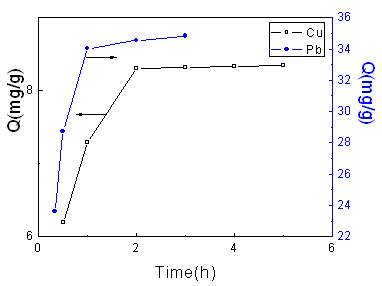
Fig.2 Effect of adsorption time on
adsorption capacity
Temperature: 25 ℃; [Cu(II)]: 1.2475 mmol/L; GP: 75.5
%.
Temperature: 25 ℃; [Pb(II)]: 1.2503 mmol/L; GP: 75.5
%.
3.2 Effect of adsorption
time
As shown in Fig.2, the adsorption capacity of Cu (II) and Pb
(II) increases with the adsorption time during the first 2 h and 1 h, respectively, and
then level off toward the equilibrium adsorption capacity. It can be ascribed to the
following facts that the adsorption process is a heterogeneous reaction, and the
complexation between Cu (II), Pb (II) and aminoethyl required a definite time to get the
adsorption equilibrium. Compared with Cu (II) ion, the adsorption of Pb (II) ion onto
starch copolymer reaches dynamic equilibrium in a shorter time, which is consistent with
the reports by Sun et al [18].
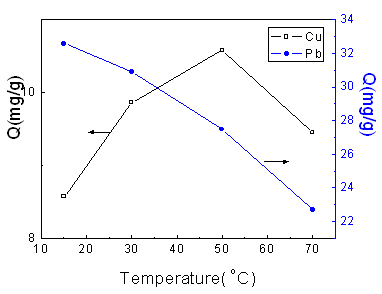
Fig.3 Effect of temperature on
adsorption capacity
[Cu (II)]: 1.2475 mmol/L; GP: 75.5 %; Adsorption time: 2 h.
[Pb (II)]: 1.2503 mmol/L; GP: 75.5 %; Adsorption time: 1 h.
Fig.3 shows the effect of the adsorption temperature on the adsorption capacities toward Cu (II) and Pb (II) ions from aqueous solution. For Cu (II), when the temperature is lower than 50oC, the solubility of the poly(aminoethyl methacrylate) on the surface of adsorbent is enhanced with the increasing of the temperature, so the content of amino group which can complex with Cu (II) ion increases stepwise; when the temperature is higher than 50oC, the desorbing plays an important role, so there is a maximum at 50oC. The adsorption capacity of Pb (II), however, drops down all the time with the temperature increasing from 15oC to 70oC. It can be well described by the work of Zhou et al that the adsorption of Pb (II) is an exothermic process, so low temperature is propitious to adsorption [19].
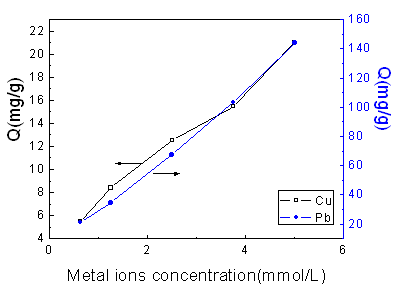
Fig.4 Effect of metal ions concentration on adsorption capacity
Cu: GP: 75.5 %; Adsorption time: 2 h; Temperature: 25oC.
Pb: GP: 75.5 %; Adsorption time: 1 h; Temperature: 25oC
3.4 Effect of metal ion concentration
As shown in Fig.4, adsorption capacity of starch copolymer towards Cu (II) and Pb (II) is
highly concentration-dependent. When the metal ions concentration increased, the
adsorption capacity is increased apparently. In fact, the opportunity of collision between
metal ions and amino groups increased with the enhancing of the metal ions concentration
under the same condition, which consequentially leads to the higher adsorption capacity of
the adsorbent. Considering the actual waste water, it is necessary to announce that lower
concentration of metal ions was employed in this work.
3.5 Effect of grafting percentage
Fig. 5 presents the effect of the grafting percentage of the starch graft copolymers on
the adsorption capacities toward Cu (II) and Pb (II) ions from aqueous solutions. As
expected, higher grafting percentage results in higher adsorption capacity. Apparently,
the more amine groups were attached to the surface of the starch graft copolymer with
higher grafting percentage, which affirmatively increase the adsorption ability toward Cu
(II) and Pb (II) ions by the stronger complexation reaction.
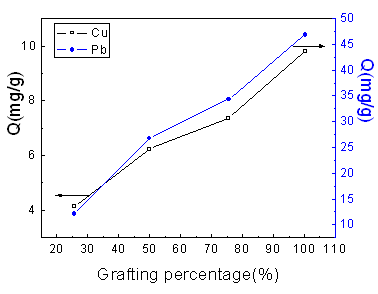
Adsorption time: 2 h; Temperature: 25oC; [Cu (II)]: 1.2475 mmol/L.
Adsorption time: 1 h; Temperature: 25 oC; [Pb (II)]: 1.2503 mmol/L. 3.6 Blank adsorption experiment of starch and desorption study
In comparison with the grafting starch copolymer, crosslinked starch by itself was also used to adsorbed Cu (II) and Pb (II) ions at the same conditions. The adsorption capacity of Cu (II) and Pb (II) onto pure starch is as low as 0.34 and 2.55 mg/g, respectively, indicating that the introduction of 2-aminoethyl methacrylate onto starch is very essential for its effective adsorption behaviors.
The desorption efficiency, as an important characteristic of adsorbents, was evaluated by desorption agents 1 N HCl at 25oC over 1 h. The desorption percentages of different grafting percentage including 27.5%, 50.0%, 75.5% and 100.3% are 94%, 96%, 95% and 96%, respectively. The metal ions are consequentially released from the graft starch copolymer due to the more affinity of HCl with the amino groups, when the acidic solution makes contact with the loaded adsorbent. Moreover, most of the amino groups are located on the particle surface, leading to the fast and complete desorption process.
4 CONCLUSIONS
Crosslinked starch graft copolymers with aminoethyl groups were prepared by grafting
2-aminoethyl methacrylate onto crosslinked starch. Their adsorption behaviors of the
starch graft copolymer toward copper (II) and lead (II) ions from aqueous solutions were
evaluated by a batch technique under various conditions. For the starch graft copolymer
with the grafting percentage of 75.5%, the saturation adsorption capacity toward copper
(II) and lead (II) ions was found to be 21.05 and 144.08 mg/g, respectively. Besides, the
metal ions concentration and grafting percentage have considerable influences on the
adsorption of copper (II) and lead (II) ions on the starch graft copolymer.
REFERENCES
[1] Xu S M, Zhang S F, Lu R W et al. J. Applied Polymer Sci., 2003, 89: 263.
[2] Xu S M, Feng S, Yue F et al. J. Applied Polymer Sci., 2004, 92: 728.
[3] Xu S M, Feng S, Peng G et al. Carbohydrate Polymers, 2005, 60: 301.
[4] Huang M R, Li X G, Peng Q Y. Gongye Shui Chuli, 2005, 25 (1): 13.
[5] Huang M R, Li X G, Li S X. Huaxue Jinzhan, 2005, 17 (2): 299.
[6] Terashima Y, Ozaki H, Sekiue M. Wat.Res, 1986, 20: 537.
[7] Boto B A, Pawlowski L. Water Treatment by Ion Exchange, Chapman and Hall, New York,
1987.
[8] Barcicki J, Pawlowski L, Cichocki A, in: L. Pawlowski (Ed.), Physicochemical Methods
for Water and Wastewater Treatment, Pergamon, London, 1980.
[9] Liu M H, Zhang X S, Deng Y. Shui Chuli Jishu, 2000, 26 (4): 222.
[10] Sun X Z, Su Z X.. Huaxue Yanjiu, 2005, 16 (1): 29.
[11] Lu W, Yang J Z, Cui C X. J. Appl. Polym. Sci., 2003, 89: 263.
[12] Xu S M, Feng S , Yue F, et al. J. Appl. Polym. Sci.,
2004, 92: 728.
[13] Xu S M, Feng S, Peng G, et al. Carbohyd. Polym., 2005, 60: 301.
[14] Guo L, Zhang S F, Ju B Z, et al. Carbohydrate Polymers, 2006, 63: 487.
[15] Yang Y, Shao J. J. Appl. Polym. Sci., 2000, 77: 151.
[16] Padilha P M, Pacha J C, Moreira J C, et al. Talanta, 1997, 45: 317.
[17] Dong Y M, Lu J M, Bao Z Y. Haerbin Gongye Daxue Xuebao, 2004, 36(9): 1161.
[18] Sun J M, Xu P, Sun H W. Fenxi Huaxue, 2004, 32(10): 1356.
[19] Zhou S Y, Wang J, Chen G L. Fujian Huagong, 2002, 1: 4.
氨乙基交联淀粉接枝共聚物对铜与铅金属离子吸附性能的研究
邓奎林,贾娜,张雅芹,阎大伟,侯段敏
(河北大学 化学与环境科学学院 保定 071002)
摘要 本文通过交联淀粉与甲基丙烯酸氨乙基酯的接枝反应制备出含氨乙基的交联淀粉接枝共聚物,并研究了该共聚物对水溶液中Cu(Ⅱ)和Pb(Ⅱ)的吸附行为。利用动态吸附方法考察了各种参数,如金属离子浓度、吸附时间、接枝百分率及吸附温度对接枝共聚物吸附能力的影响。Cu(Ⅱ)和Pb(Ⅱ)达到吸附平衡的时间分别为2 h和1 h,吸附量随接枝百分率及金属离子浓度的增加而增大。当金属离子浓度约为5.0 mmol/L时,接枝百分率为75.5%的接枝共聚物对Cu(Ⅱ)和Pb(Ⅱ)的饱和吸附量可达21.05 mg/g和144.08 mg/g,并且其解吸附率达到95%以上。
关键词 吸附;重金属离子;淀粉接枝共聚物;污水处理