| http://www.chemistrymag.org/cji/2006/08b069ne.htm | Nov. 1,
2006 Vol.8 No11 P.69 Copyright |
Solvent-free synthesis of 4H-benzo[b]pyran derivatives catalyzed by NaOAc and PEG400
Cao Yuqing, Li YaBin, Wu
Guoqiang
(College of Pharmacy, Hebei University, Baoding 071002, China)
Received Sep. 24, 2006.
Abstract 4H-benzo[b] pyran derivatives
were synthesized by one-pot, three-component reaction of aromatic aldehyde, active
methylene compounds, and 1,3- cyclohexanedione in good yields using sodium acetate and
PEG400 as catalyst under solvent-free conditions.
Keywords 4H-benzo[b] pyran, 1,3-cyclohexanedione, PEG400, solvent-free
reaction
1. INTRODUCTION
4H-benzopyran derivatives have attracted great attention recently in synthetic organic
chemistry due to their wide range of biological activity and pharmacological property,
such as anti-coagulant, anti-cancer, spasmolytic, diuretic, and anti-ancaphylactin [1].
Literatures reported syntheses of such compounds in organic solvent or ionic
liquid [2,3]. These methods have some shortcomings in terms of poor
yields, environmental pollution and expensive solvent, not easy work-up. Therefore, the
improvements in such synthesis had been sought continuously [4-7]. It also has
been reported that these compounds were synthesized by microwave irradiation [8].
Although microwave irradiation in organic reactions is applicable in the laboratory, and
it cannot be widely applied in industry.
The demand for increasing clean and efficient chemical synthesis is
continuously becoming more urgent from both an economic and an environmental standpoint.
Organic synthesis in the absence of solvent has been received much attention because of
several advantages in preparative procedures, such as environmental compatibility, easy
work-up, enhanced selectivity, reduction of by-products, and much improved reaction rates
[9-11]. These would be especially important during industrial production.
Polyethylene glycols
(PEGs) can be regarded as the acyclic ether and have been used as PTC in many organic
reactions owing to their stability, low cost, poisonlessness, and easy availability
[12,13]. PEG400 was more suitable for solid-liquid phase solvent-free reactions. Our
laboratory has reported
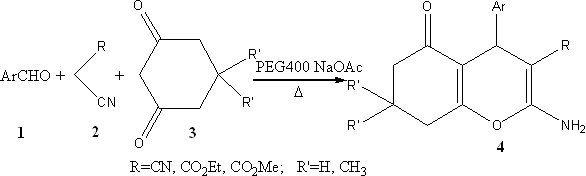
Scheme 1
2. RESULTS AND DISCUSSION
In order to determine the optimum reaction
conditions for the synthesis of 4H-benzopyran derivatives in fast and more efficient way,
we have studied the efficiency of different bases. Using NaOAc, NaOH, KF,
MgO and CaO as catalyst for the reaction of p-nitrobenzaldehyde with 1,
3-cyclohexanedione and malononitrile, the yields of 88%, 74%, 57%, 51%, and 55%
respectively, were obtained under the same reaction conditions. The better yields were
obtained when sodium acetate
Table 1. Preparation of 4H-benzopyran derivatives under solvent-free conditions.
Entry |
Ar |
R |
R' |
Time
|
Yield
|
M.p |
M.p (Lit) |
4a |
4-ClC6H4 |
CN |
H |
3 a |
87 |
224-227 |
226-229[5] |
4b |
2-ClC6H4 |
CN |
H |
3 a |
92 |
212-214 |
213-215[5] |
4c |
4-CH3OC6H4 |
CN |
H |
6 a |
83 |
194-196 |
193-195[5] |
4d |
4-NO2C6H4 |
CN |
H |
2.5 a |
88 |
230-233 |
234-235[5] |
4e |
3-NO2C6H4 |
CN |
H |
3 a |
89 |
197-199 |
198-200[5] |
4f |
2,4-Cl2C6H3 |
CN |
H |
3 a |
90 |
223-224 |
225-227[5] |
4g |
3,4-OCH2OC6H3 |
CN |
H |
4.5 a |
85 |
211-213 |
211-214[5] |
4h |
C6H5 |
CO2Et |
CH3 |
2 b |
88 |
161-163 |
158-160[2 |
4i |
4-ClC6H4 |
CO2Et |
CH3 |
2 b |
82 |
149-150 |
150-152[2] |
4j |
2-ClC6H4 |
CO2Et |
CH3 |
2.5 a |
85 |
180-182 |
181-183[2] |
4k |
3-NO2C6H4 |
CO2Et |
CH3 |
2.5 a |
85 |
179-181 |
180-182[8 |
4l |
3,4-(CH3O)2C6H3 |
CO2Et |
CH3 |
3 b |
75 |
153-156 |
155-157[8] |
4m |
4-FC6H4 |
CO2Et |
CH3 |
2 b |
87 |
153-154 |
152-155[3] |
4n |
3-BrC6H4 |
CO2Et |
CH3 |
2 b |
82 |
133-134 |
133-135[3] |
4o |
4-BrC6H3 |
CO2Et |
CH3 |
3 b |
85 |
158-160 |
160-162[8] |
4p |
4-CH3C6H4 |
CO2Et |
CH3 |
2.5 b |
80 |
153-156 |
156-157[2] |
4q |
C6H5 |
CO2Me |
CH3 |
2.5 b |
83 |
145-146 |
146-148[2] |
4r |
4-ClC6H4 |
CO2Me |
CH3 |
2 b |
85 |
164-166 |
167-168[2] |
4s |
4-CH3C6H4 |
CO2Me |
CH3 |
3 b |
70 |
169-172 |
172-174[2] |
4t |
3,4-(CH3O)2C6H3 |
CN |
CH3 |
3 a |
80 |
173-174 |
170-173[2] |
4u |
4-NO2C6H4 |
CN |
CH3 |
2 b |
89 |
127-130 |
130-132[2] |
4v |
4-FC6H4 |
CN |
CH3 |
2 a |
93 |
182-184 |
184-186[3] |
bThe reactions temperature were 130oC
cIsolated and unoptimized yields
From the data in the Table
1, the reaction of aromatic aldehydes with active methylene compounds and
1,3-cyclohexanedione under solvent-free conditions provided the corresponding 4H-benzo[b]
pyran derivatives in satisfactory yields. It has been found that the reaction of aldehydes
with electron withdrawing groups such as -Cl and -NO2 in the aromatic ring, with active methylene
compounds and 1,3-cyclohexanedione can be carried out in relatively shorter time and high
yield than with electron donating group such as -OCH3.
The yield of 4H-benzopyran bearing chloro group at para position on the aryl ring is lower
than that of the 4H-benzopyran bearing chloro group at ortho position on the aryl ring.
The reaction rate would slow down along with increasing amount of the high melting point
of products, so a high reaction temperature was needed. However, high temperature will
result in low yield especially for the ester bond due to the oxidation and hydrolysis of
some reactant and product by water generating in reaction. Higher reaction temperature is
necessary for the reactions of the products with the high melting point in order to assue
the reaction mixture in liquid state.
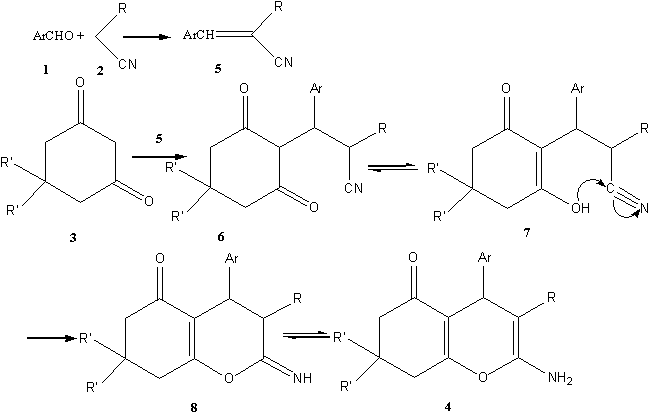
We consider the reaction to proceed via aldol condensation, addition,
enolisation, cyclodehydration and tautomerisation (Scheme 2.). Firstly, compound (5)
was obtained by aromatic aldehyde reaction with active methyl compounds via Knoevenagel
reaction. Secondly, Compound (5) reacted with the electrophilic C=C double bond
giving the intermediate (6). Then the intermediate (6) was cyclized by the
nucleophilic attack of OH group on the cyano (CN) moiety and gave the intermediate (7).
Finally the expected products (4) were obtained by isomerization (7→8→4).
In summary, we have developed a safe, environment-compatible and easy work-up method for the synthesis of benzopyran derivatives from substituted aromatic aldehydes, active methylene compounds and 1, 3-cyclohexanedione or 5, 5-dimethyl-1, 3-cyclohexanedione in the presence of PEG400 and sodium acetate under solvent-free conditions.
3. EXPERIMENTAL
TLC was GF254 thin layer chromatography with petroleum ether/ethyl acetate
as eluent. Aromatic aldehydes, active methylene compounds and 1,3-cyclohexanedione were
obtained from commercial suppliers and not purified. Melting points were determined on a
microscopy apparatus and are uncorrected.
General Procedure for the Synthesis of 4H-benzo[b]pyrans (4)
A mixture of aromatic aldehyde 1
(0.1mol), active methylene compounds 2 (0.11mol), 1,3-cyclohexanedione 3
(0.11mol), NaOAc (0.01mol), and PEG400 (0.005mol) were taken
into a 50ml three-necked, round-bottomed flask equipped with drying tube filled with
KOH. The mixture was vigorously stirred and heated at the assigned temperature for a
period of time as required to complete the reaction (monitored by TLC). Then the hot
mixture was poured into a breaker and cooled to room temperature and washed with water.
The solid was obtained by filtration. The crude products were purified by
recrystallization from ethanol (95%).
REFERENCES
[1] Andreani L L, Lapi E. Bull. Chim. Farm, 1960, 99: 583-586.
[2] (a) Wang X S, Shi, D Q, Tu S J., et al. Synth Commun, 2003, 33; 119.
(b) Kamaljit S, Jasbir S. Tetrahedron, 1996, 52:
14273-14280.
[
[4] Jin T S, Wang A Q, Zhang J S., et al. (Chin. J. Org.Chem), 2004, 24: 1598-1600.
[5] Shi D Q, Mou J, Zhuang Q Y., et al. Journal Chem Res (s), 2004, (12): 821-823.
[6] Ipsita Devi, Pulak J B. Tetra.Lett, 2004, 45: 8625 –8627.
[7] Gao Y, Guo C. (Chin. J. Appl.Chem), 2002, 19: 401-402.
[8] Tu S J, Wang H, Feng J Q, et al. Synth. Commun, 2001, 31(17): 2663-2666.
[9] Tanaka K, Toda F. Chem. Rev, 2000, 100: 1025.
[10] Loupy A. Top. Curr. Chem, 2000, 206: 153;
[11] Lonpy A, Song S J, Sohn S M, et al. J. Chem.Soc, Perkin Trans, 2001, (1): 1220.
[12] Wang X C, Li Z, Da Y X, et al. Synth. Commun, 1999, 29: 4153.
[13] Li P, Alper H. J. Org. Chem, 1986, 51: 4351-4356.
[14] Wang M, Chang K R. Ind. Eng. Chem. Res, 1990, 29 (1): 40
[15] (a) Cao Y Q, Du Y F, Li J T. J. Chem. Res. (s), 2003, (8): 500-501.
(b) CaoY Q, Dai Z, Zhang R. Synth. Commun, 2005, 35: 1045-1049.
无溶剂下PEG400和无水醋酸钠一锅法催化合成4H-苯并吡喃衍生物
曹玉庆 李亚彬 吴国强
(河北大学药学院,071002, 保定)
摘要 在无溶剂条件下,由PEG400和无水醋酸钠催化的芳香醛,活泼亚甲基化合物,和1,3-环己二酮三种化合物一锅法合成苯并吡喃衍生物取得了较好的收率。
关键词 苯并吡喃,1,3-环己二酮, 聚乙二醇400,
无溶剂