http://www.chemistrymag.org/cji/2007/091003ne.htm |
Jan. 2,
2007 Vol.9 No.1 P.3 Copyright |
Yang
Wenzhi, Li Haiying, Zhang Hongle
(College of Pharmacy, Hebei University, Baoding 071002, China)
Received Nov. 26, 2006.
Abstract Synthesis of 4H-chromene-3-carbonitrile derivative was carried out conveniently using NaOH and KF/Al2O3 as catalyst under ultrasonic irradiation. The reactions are completed within 5 min to give satisfactory yields. KF/Al2O3 was more effective catalyst in one-pot reaction.Keywords: a,a'-dibenzylidenecyclohexanone; malononitrile; 4H-chromene-3-carbonitrile derivative; potassium fluoride supported on alumina
1 INTRODUCTION
The pyridine derivatives have shown important biological activities as pharmaceuticals
and potential agrochemicals such as herbicides, and pyrans ring can be
transformed to pyridine systems related to pharmacological important calcium antagonists
of the DHP type.[1-3] It was also reported previously that when a,a'-dibenzylidenecyclohexanone was
mixed with malononitrile using different solvent and basic catalysts under microwave
irradiation, 4H-quinoline-3-carbonitrile derivatives were obtained in excel yields and
4H-chromene-3-carbonitrile derivatives were in mild yields respectively.[4] Our
laboratory has reported the Claisen-Schmidt condensation of cyclohexanone with
benzaldehyde catalyzed by KF/Al2O3 under refluxed methanol, and the
results are better than that under conventional heating condition.[5] In the
present communication, the results on the KF/Al2O3-catalyzed
reaction between a,a'-dibenzylidenecyclohexanone
and malononitrile in different reaction conditions are reported.
2 EXPERIMENTAL
a,a'-dibenzylidenecyclohexanone
was synthesized according to the literature.[5] Melting points were
uncorrected. 1H NMR spectra were measured on Bruker AM-400S (400 MHz)
spectrometer using TMS as internal standard and CDCl3 as solvent. Sonication
was performed in a KQ-500B ultrasonics cleaner with a frequency of 25 kHz and a normal
power of 500 W. The reaction flask was located on the maximum energy area in the cleaner
and addition or removal of water was used to control the temperature of the water bath.
Compound 3a.
2-Amino-8-benzylidene-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile: white needle,m.p. 229-231 oC (lit 228-230 oC)[4];
1H NMR (CDCl3,400 MHz) dH:1.58 ~ 1.69 (2H, m, CH2), 1.96 ~ 2.07 (2H, m, CH2),
2.60 ~ 2.78 (2H, m, CH2), 3.99 (1H, s, CH), 4.53 (2H, s, NH2),
6.90 (1H, s, CH), 7.27 ~ 7.40 (10H, m, ArH).
2.1 Preparation of the catalyst (KF-solid supports)
Anhydrous potassium fluoride (20 g) was dissolved in distilled water (80 ml) and
mixed with solid supports (30 g), such as neutral alumina or molecular sieve respectively.
The mixture was stirred at 65–75oC for 1
h. The water was removed under reduced pressure. The resulting free flowing powder was
dried at 120oC for 4 h. The content of KF is about 30% (150mg mixture/mmol KF).
2.2 General procedure
The a,a'-dibenzylidenecyclohexanone
(0.5 mmol) and malononitrile (0.6 mmol) are mixed with MeOH or EtOH (20-30 mL). KF-Al2O3
(75 mg) or NaOH is added. The mixture was irradiated in the water bath of an
ultrasonic cleaner at the temperature for the period as indicated in Table (Sonication
was continued until crystals were appeared or a,a'-dibenzylidenecyclohexanone was disappeared indicated by TLC). The
catalyst was removed by filtration and washed with boiled ethanol or 70% ethanol. The
solvent was evaporated under reduced pressure and the residue was crystallized with boiled
EtOH to give 4H-chromene-3-carbonitrile derivative. The authenticity of the products was
established by comparing their melting points with that in the literature and data of m.p. and 1H NMR spectra.[4]
3 RESULTS AND DISCUSSION
Zhou[4] reported a synthesis of 4H-quinoline-3-carbonitrile derivatives by
using NaOH in MeOH under microwave irradiation, while by using piperidine in EtOH under
microwave irradiation, 4H-chromene-3-carbonitrile derivatives were obtained. The results
stated above spur us to study the possibility of synthesis of 4H-quinoline-3-carbonitrile
or 4H-chromene-3-carbonitrile derivatives (3) in different solvent and catalysts under
ultrasonic irradiation. Herein, we wish to report the results of the reaction of a,a'-dibenzylidenecyclohexanone (1) and
malononitrile (2) under ultrasonic irradiation (Scheme 1). As shown in Scheme 1 and Table
1, a,a'-dibenzylidenecyclohexanone
(1) and malononitrile (2), was transferred to its 4H-chromene-3-carbonitrile derivative,
when it was treated with different catalysts and solvent under irradiation of ultrasonic
to only give one product (3a) in excellent yields. Two kinks of catalyst, NaOH and KF/Al2O3
were investigated in this reaction. The catalyst plays a crucial role in the success of
the reaction in terms of the rate and the yields. KF/Al2O3 was more
effective in this reaction, product (3a) gave out 80% yield in MeOH and 95% yield in EtOH
within 5 min under ultrasonic irradiation respectively. In contrast, 3a obtained 67% yield
in MeOH and 86% yield in EtOH using NaOH-catalysis at the same reaction condition.
Irradiation time significantly affected the yields. Thus,
whether using KF-Al2O3 or NaOH, the
reaction time up to 5 min enhance the yield but not improve the yield by prolonged
irradiation after 5 min.
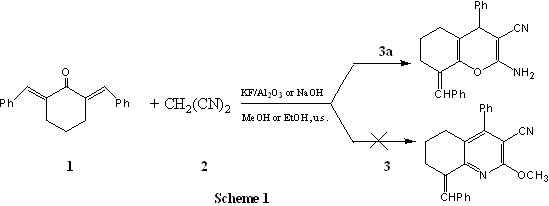
Table 1 The reaction between a,a'-dibenzylidenecyclohexanone and malononitrile catalyzed by KF-Al2O3
or NaOH.
Compound |
Reaction condition |
Cat. |
Time(min)/ Temperature (oC) |
yield /%, (lit.) |
m.p./oC (lit.) |
3a |
r.t./MeOH |
NaOH |
180/25 |
69 |
229-231/228-230[4] |
360/25 |
74 |
||||
u.s./MeOH |
NaOH |
5/25 |
67 |
||
15/25 |
58 |
||||
30/25 |
57 |
||||
KF/Al2O3 |
5/25 |
80 |
|||
10/25 |
70 |
||||
r.t./EtOH |
NaOH |
1080/30 |
94 |
||
u.s. /EtOH |
NaOH |
5/30 |
86 |
||
15/30 |
82 |
||||
30/30 |
83 |
||||
KF/Al2O3 |
5/30 |
95 (70[4], 81[6]) |
A
The molar ratio of a,a'-dibenzylidenecyclohexanone to catalyst, mixture of a,a'-dibenzylidenecyclohexanone: malononitrile=1:1.2 mol/mol We can also observe
that the reaction takes place slowly without sonication at room temp. It was necessary to
stir the mixture using NaOH-catalysis at 1080 min in EtOH and obtain 95% yield, a similar
yield using KF-Al2O3-catalysis that obtained at 30 oC after 5 min of
sonication time. From the Table 1, The reaction of a,a'-dibenzylidenecyclohexanone (1)
with malononitrile (2) gave 95% yield of 3a catalyzed by KF-Al2O3
in 5 min under ultrasound irradiation, and the yield 3a is higher than that
obtained 70% yield catalyzed by piperidine-ethanol in 5 min under microwave irradiation,[4]
and also higher than 81% yield catalyzed by KF-Al2O3 in DMF within
10-14h at room temperature.[6]
KF-Al2O3 catalysis usually gave better
yields, simpler reaction conditions, successfully reused,[5] and easier product
mixture nonaqueous workup than NaOH. Compared with the product separation from the
unreacted substrates, crystallized from ethanol, when using NaOH the disposing of process
was more tedious.
4 CONCLUSION
It was concluded that the best synthetic conditions of the 4H-chromene-3-carbonitrile
derivative (3b) used the mole ratio of KF/Al2O3 to a,a'-dibenzylidenecyclohexanone (1/1) in
ethanol under ultrasonic irradiation for 5 min, and the yield of 3a was up
to 95%.
ACKNOWLEDGEMENT The work were supported by the Natural Science Instruction Project of Hebei Province and Natural Science Pre-Research Foundation of Hebei University
REFERENCES
[1] Temple C, Rener G A, Waud W R et al. J. Med. Chem., 1992, 35: 3686
[2] Marco J L, Martin N, Grau A M et al. Tetrahedron, 1994, 50: 3509
[3] Albert D A, Cano F H, Martin N et al. J. Chem. Soc. Perkin Trans(I), 1997, 3401
[4] Zhou J F. Synth. Commun., 2003, 33(1): 99
[5] H.Y. Li, W. Z. Yang, J. B. Yang et al; J. Chem. Res., 2004, 11, 744-746; Yang W Z, Li
H Y, Wang S X et al. Chemical Journal on Internet, 2004, 6(4): 25
[6] Wang X S, Shi D Q, Du Y, et al. Synth. Commun., 2004, 34(8), 1425-1432
杨文智,李海鹰,张红乐
(河北大学药学院,河北 保定 071000)
摘要在超声辐射下,分别使用NaOH 和KF/Al2O3催化a,a'-二苯叉环己酮与丙二腈反应,反应5 min,合成较好产率的4H-苯并吡喃-3-腈衍生物。KF/Al2O3是催化此反应的良好催化剂。
关键词 a,a'-二苯叉环己酮;丙二腈;4H-苯并吡喃-3-腈衍生;氧化铝固载氟化钾