http://www.chemistrymag.org/cji/2007/096025pe.htm |
Jun 10,
2007 Vol.9 No.6 P.25 Copyright |
Molecular spectroscopic studies on the interaction between oxaprozin-P and bovine serum albumin
Sun
Shaofa, Xiang Guangya#
(Department of Chemistry and Life Science, Xianning College, Xianning 437000; #School
of Pharmacy, Tongji Medical College, Huazhong University of Science and Technology, Wuhan
430015)
Abstract The interaction between a kind
of NO-releasing oxaprozin(Oxaprozin-P)and bovine serum albumin (BSA) was studied by
spectroscopic methods including fluorescence and UV-vis absorption spectroscopy. The
quenching mechanism of fluorescence of BSA by Oxaprozin-P was discussed to be a dynamic
quenching procedure. The number of binding sites n and apparent binding constant Kb
was measured by fluorescence quenching method. The thermodynamic parameters, such as DH, DG and DS etc., were calculated. The results indicate that the
binding reaction was mainly entropy-driven and hydrophobic forces played major role in the
reaction. The distance r between donor (BSA) and acceptor (Oxaprozin-P) was obtained
according to Förster theory of non-radioactive energy transfer.
Keyword Oxaprozin-P; fluorescence spectroscopy; UV-vis absorption spectroscopy;
thermodynamic parameters; bovine serum albumin.
1. INTRODUCTION
Nitric oxide (NO) is an important messenger moleculer and has wide biological activity
[1]. Furoxans are widely used as nitric oxide donor in new drugs development.
Oxaprozin [2] is one of the most widely used Nonsteroidal antiinflammatory
drugs (NSAIDs) in
clinic, but as most of the NSAIDs, its side effect, especially gastric or intestinal
ulceration, limits its application. According to symbiotic approach principle, the hybrids
by coupling nitric oxide-releasing group Furoxan with Oxaprozin may greatly reduce its
side effects. Therefore, Oxaprozin-P (Fig.1) have been synthesized by our group.
It has been shown that the distribution, free concentration and the
metabolism of various drugs may be strongly affected by drug-protein interactions in the
blood stream. This type of interaction can also influence the drug stability and toxicity
during the chemotherapeutic process. Serum albumins are the most abundant proteins in
plasma. As the major soluble protein constituents of the circulatory system, they have
many physiological functions. They contribute to colloid osmotic blood pressure and are
chiefly responsible for the maintenance of blood pH [3], So they can play a
dominant role in drug disposition and efficacy [4]. Many drugs and other small
bioactivity molecules bind reversibly to albumin and other serum components that then
function as carriers. Serum albumin often increases the apparent solubility of hydrophobic
drugs in plasma and modulates their delivery to cells in-vivo and in-vitro. Consequently,
it is important to know the affinity of a drug to serum albumin, even if it is not the
only factor to predict serum concentrations of the free drug.
Fluorescence and UV-vis absorption spectroscopy are powerful tool for
the study of the reactivity of chemical and biological systems since it allows
non-intrusive measurements of substances in low concentration under physiological
conditions. In the present work, the interaction between Oxaprozin-P and BSA by
fluorescence and UV-vis absorption spectroscopy was demonstrated. The effect of the energy
transfer was studied according to fluorescence resonance energy transfer (FRET). The aim
of this work is to determine the affinity of Oxaprozin-P to BSA, and to investigate the
thermodynamics of their interaction. To resolve this problem, the UV-vis and fluorescent
properties of Oxaprozin-P, as well as BSA were investigated.
Figure 1. Molecular structure of Oxaprozin-P
2. EXPERIMENT
2.1 Materials
Oxaprozin-P was synthesized and characterized by the Group of Organic Synthesis, Pharmacy
School of Tongji Medical College, Huazhong University of Science and Technology, P. R.
China. BSA, electrophoresis grade reagents, was obtained from Sigma. Tris-Base had a
purity of no less than 99.5% and NaCl, HCl, etc. were all of analytical purity. The
samples were dissolved in Tris-HCl buffer solution (0.05 mol·L-1
Tris-Base, 0.10 mol·L-1 NaCl, pH = 7.4). The bovine serum albumin
solutions were prepared 30 minutes before experiment. All solutions were used with doubly
distilled water.
2.2. Apparatus
All fluorescence spectra were recorded on F-2500 Spectrofluorimeter in the ratio mode with
temperature maintained by circulating bath (Hitachi, Japan); TU-1901 spectrophotometer
(Puxi Ltd. of Beijing, China) was used for scanning UV¨Cvis spectra; the mass of the sample was accurately weighed using a
microbalance (Sartorius, ME215S) with a resolution capacity of 0.1 mg.
2.3. Spectroscopic measurements
The absorption spectra of BSA, Oxaprozin-P and their mixture were performed at room
temperature. The fluorescence measurements were performed at different temperatures (298,
302 and 310 K). Excitation wavelength was 285 nm. The excitation and emission slit widths
were set at 2.5 nm. Appropriate blanks corresponding to the buffer were subtracted to
correct background fluorescence.
3. RESULTS AND DISCUSSIONS
3.1 Fluorescence characteristics of BSA and Oxaprozin-P
Fluorescence quenching refers to any process which decreases the fluorescence intensity of
a sample. A variety of molecular interactions can result in quenching. These include
excited-state reactions, molecular rearrangements, energy transfer, ground-state complex
formation, and collisional quenching.
For collisional quenching, the decrease in intensity is described by
the well-known Stern-Volmer equation as follows [5]:
![]() (1)
(1)
Where, F0 and F are the steady-state
fluorescence intensities in the absence and in the presence of quencher, KSV
is the Stern-Volmer quenching constant, and [Q] is the concentration of
quencher respectively.
Figure 2 shows the emission spectra of BSA in the presence of various
concentrations of Oxaprozin-P. It is apparent that the fluorescence intensity of BSA
decreased regularly with the increasing of Oxaprozin-P concentration. The experiment was
carried out within the linear part of Stern-Volmer dependence (F0/F
against [Q]), and stabilized the concentrations of BSA at 1.0¡Á10-5 mol·L-1,
the concentration of Oxaprozin-P varied from 0 to 4.0¡Á10-5 mol·L-1
at the step of 0. 5¡Á10-5 mol·L-1.
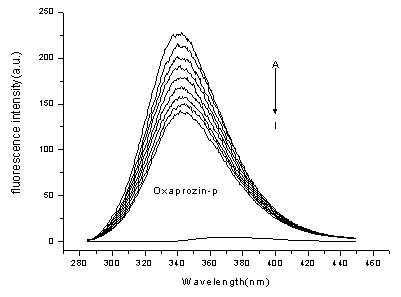
Figure 2. Emission spectra of BSA in the
presence of various concentrations of Oxaprozin-P (T=298K)
[BSA]= 1.0¡Á10-5 mol·L-1; [Oxaprozin-P] / (10-5
mol·L-1), A-I: 0; 0.5; 1.0; 1.5; 2.0; 2.5; 3.0; 3.5; 4.0.
curve K shows the emission spectrum of Oxaprozin-P only(lex=370nm), [Oxaprozin-P] = 1.0¡Á10-5 mol·L-1.
Quenching can occur
through different mechanisms, which usually classified as dynamic quenching and static
quenching. Dynamic and static quenching can be distinguished by their different dependence
on temperature and viscosity. Higher temperatures result in faster diffusion and hence
larger amounts of collisional quenching. Higher temperatures will typically result in the
dissociation of weakly bound complexes, and hence smaller amounts of static quenching.
Figure 3 displays the Stern-Volmer plots of the quenching of BSA
tryptophan residues fluorescence by Oxaprozin-P at different temperatures. The
corresponding Stern-Volmer quenching constant at different temperatures are shown in Table
1.
These results indicate that the probable quenching mechanism of
fluorescence of BSA by Oxaprozin-P is a dynamic quenching procedure, because the KSV
increased with the temperature rising.
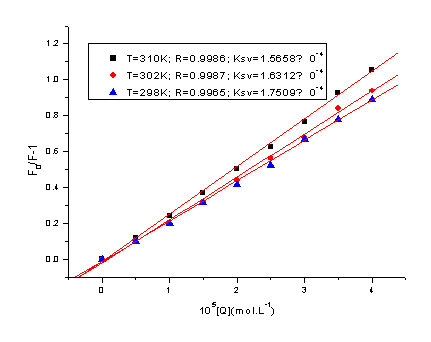
Figure 3. Stern-Volmer plot at three
different temperatures
Table 1. Stern-Volmer quenching constant KSV and relative thermodynamic parameters
pH |
T |
10-4Ksv |
Ra |
SDb |
DH/
kJ·mol-1 |
DG/
kJ·mol-1 |
DS/ J·mol-1·K-1 |
7.4 |
298 |
1.5658 |
0.9985 |
0.015 |
7.12 |
- 1.11 |
27.6 |
302 |
1.6312 |
0.9977 |
0.019 |
- 1.22 |
|||
310 |
1.7509 |
0.9965 |
0.026 |
- 1.44 |
aR is the linear correlated coefficient; b SD is standard deviation.
3.2 Thermodynamic parameters and
nature of the binding forces
The interactions forces between a drug and biomolecule may include hydrophobic force,
electrostatic interactions, van der Waals interactions, hydrogen bonds, etc. The
Stern-Volmer quenching constants of BSA were measured at three different temperatures
(298, 302 and 310 K). The slope of a plot of the logarithum of bimolecular quenching
constant vs. 1/T (T, absolute temperature) are linear within
experimental error, which allows one to calculate the energy change for the quenching
process [6]. If the enthalpy change (DH) does not vary significantly over the temperature range
studied, then its value and that of entropy change (DS) can be determined from the Van't Hoff equation:
![]() (2)
(2)
Where constants K are analogous to the Stern-Volmer quenching
constants KSV at the corresponding temperature and R is
the gas constant [7] (the temperatures used were 298, 302 and 310 K). The free
energy change (DG)
is estimated from the following relationship:
DG=DH - TDS (3)
Figure 4, by fitting the data of Table 1, shows that the assumption of
near constant DH
is justified. Table 1 shows the values of DH and TDS obtained for the binding site from the slopes and
ordinates at the origin of the fitted lines.
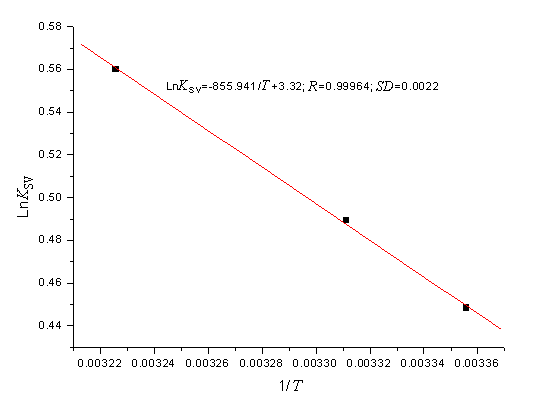
Figure 4. Van't Hoff plot, pH 7.40,
[BSA]=1.0¡Á10-5 mol·L-1
From Table 1, it can be
seen that the negative sign for free energy (DG) means that the interaction process is spontaneous. The
positive enthalpy (DH)
and entropy (DS)
values of the interaction of Oxaprozin-P and BSA, indicate that the binding is mainly
entropy-driven and the enthalpy is unfavorable for it, the hydrophobic forces played major
role in the reaction [7,8].
3.3 Analysis of binding equilibria
When small molecules bind independently to a set of equivalent sites on a macromolecule,
the equilibrium between free and bound molecules is given by the equation below [9]:
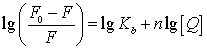 (4)
(4)
Where, in the present case, Kb is the binding
constant to a site, and n is the number of binding sites per BSA [10].
Table 2 gives the results at different temperatures analyzed in this
way for BSA. The linear correlated coefficients (R) are larger than 0.999, and the
standard deviation is less than 0.02, indicating that the assumptions underlying the
derivation of equation (4) are satisfied. Table 2 shows the results of Kb
decreased slightly with the temperatures rising, which may indicates that there is
Oxaprozin-P molecular binding with BSA and forming an unstable compound. The unstable
compound would be partly decomposed when the temperature rising, therefore, the values of Kb
decreased slightly with the temperatures rising. Table 2 shows the number of binding sites
n approach 1£¬we can conclude that Oxaprozin-P
molecule formed 1:1 compound with BSA molecule.
Table 2. Binding constant Kb and binding sites n at different temperatures
pH |
T |
10-4Kb
|
n |
Ra |
SDb |
7.4 |
298 |
9.7904 |
1.125 |
0.9996 |
0.0112 |
302 |
6.6984 |
1.144 |
0.9994 |
0.0131 |
|
310 |
5.3029 |
1.176 |
0.9992 |
0.0160 |
a R is the linear correlated coefficient; b SD is standard deviation
3.4 Energy transfer between BSA and
Oxaprozin-P
The Förster theory of molecular resonance energy transfer [11] points out:
in addition to radiation and reabsorption, a transfer of energy could also take place
through direct electrodynamic interaction between the primarily excited molecule and its
neighbors. According to this theory, the efficiency of energy transfer between the donor
and acceptor, E, could be calculated by the following equation [12]:
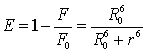 (5)
(5)
Where r is the distance between the donor and acceptor [13], and R0 is the critical distance when the efficiency of transfer is 50%.
![]() (6)
(6)
In equation (6), K2 is the orientation factor related to the geometry of the donor and acceptor of dipoles and K2= 2/3 [7] for random orientation as in fluid solution; N is the refracted index of medium; f is the quantum yield of the donor in the absence of acceptor; J expresses the degree of spectral overlap between the donor emission and the acceptor absorption (Fig. 6), which could be calculated by the equation(7):
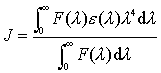 (7)
(7)
Where, F(l) is the corrected fluorescence intensity of the donor in the wavelength range l to l+Dl; e(l) is the extinction coefficient of the acceptor at l. In the present case, N = 1.36, f = 0.15 [14], according to the equation (5) ~ (7), we could calculate that R0 = 3.49 nm; E = 0.11 and r = 4.94 nm. The donor-to-acceptor distance r©‚8 nm [15], and 0.5R0 < r< 1.5R0 [16], indicate that the energy transfer from BSA to Oxaprozin-P occurs with high possibility.
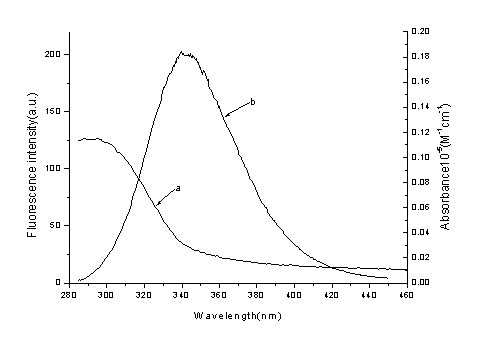
Figure 5. Spectral overlap
of Oxaprozin-P absorption (a) with BSA fluorescence (b)
[BSA] = [Oxaprozin-P] =1.0¡Á10-5 mol·L-1
4. CONCLUSIONS
The main purpose of this research is to study the binding properties between BSA and
Oxaprozin-P for the great importance in pharmacy, pharmacology and biochemistry. The
interaction of Oxaprozin-P with BSA was studied by spectroscopic methods including
fluorescence spectroscopy and UV-visible absorption spectroscopy. The experimental results
also indicate that the probable quenching mechanism of fluorescence of BSA by Oxaprozin-P
is a dynamic quenching procedure, and the binding reaction is mainly entropy-driven and
hydrophobic interactions played major role in the reaction.
ACKNOWLEDGEMENT The authors gratefully acknowledge financial support of Natural Science Foundation of Hubei Province(No.2006ABA333) and Science Foundation of Hubei Provincial Educational Department (No.D200528003)
REFERRENCE
[1] J E Keeble, P K Moore. British J. of Pharmacology, 2002,137: 295-310.
[2] X Xue, W D Wang, Y H Zhang. J. China Pharmaceutical University, 2002, 33: 548-551.
[3] R E Olson, D D Christ. Ann. Rep. Med. Chem., 1996, 31: 327-337.
[4] X M He, D C Carter. Nature, 1992, 358: 209-215.
[5] J R Lakowicz, Principles of Fluorescence Spectroscopy. New York: Plenum Press, 1999:
237-265.
[6] J R Lakowicz, G Weber. Biochemistry, 1973, 2: 4161-4170.
[7]Y J Hu, Y Li, J B. Wang et al. J. Pharma. and Biomed. Anal., 2004, 36: 915-919.
[8] D P Ross, S Subramanian. Biochemistry, 1981, 20: 3096-3102.
[9] Y J Hu, Y Liu, A X Hou et al. Acta Chim. Sinica, 2004, 62: 1519-1523.
[10]Y J Hu, Y Liu, X S Shen et al. J. Molecular Structure, 2005, 738:143-147.
[11] T F?rste. Ann. Phys., 1948, 2: 55-75.
[12] L A Sklar, B S Hudson, R D Simoni. Biochemistry, 1977, 16: 819-828.
[13] J Tian, J Liu, X Tian et al. J. Mol. Struct., 2004, 691: 197-202.
[14] L Cyril, J K Earl, W M Sperry. Biochemists¡¯ Handbook.
London: E. & F. N. Spon, 1961: 84.
[15] B Valeur, J C Brochon. New Trends in Fluorescence Spectroscopy. Berlin: Springer
Press, 1999: 25.
[16] B Valeur. Molecular Fluorescence: Principles and Applications. New York: Wiley Press,
2001:250.
¡¡