http://www.chemistrymag.org/cji/2007/096027pc.htm |
Jun.10,
2007 Vol.9 No.6 P.27 Copyright |
Zhang Shuwen, Wei Baojun , Hao Pengpeng, Ba
Xinwu
(College of Chemistry and Environmental Science, Hebei University, Baoding 071002, China)
Abstract 3-acetyl-1-p-methoxyphenyl-1,4-pentanedione 1 was synthesized by the reaction of acetylacetone with
w-bromo-p-methoxyacetophenone. Subsequently, the Paal-knorr reactions of 1 with different diamine 2 gave the N,N′-Substituted bis(3-acetyl-2-methyl-5-p-methoxyphenyl) pyrroles 3a-3h. The structures of products were characterized by IR, 1H NMR spectra and elemental analysis.Meanwhile, we did preliminary research for affacted factor of synthesis.Keywords 3-acetyl-1-p-methoxyphenyl-1,4-pentanedione; Paal-knorr reaction; diamine; pyrrole
N,N'-二取代(3-乙酰基-2-甲基-5-对甲氧基苯基)吡咯的合成及表征
张书文,魏保君,郝鹏鹏,巴信武
(河北大学化学与环境科学学院;中国河北保定 071002)
2007年3月28日收稿
摘要 以乙酰丙酮和
w-溴-p -甲氧基苯乙酮为原料合成了3-乙酰基-1-对甲氧基苯基-1,4-戊二酮。3-乙酰基-1-对甲氧基苯基-1,4-戊二酮和不同的二胺通过 Paal-Knorr 反应,合成了系列N,N'-二取代(3-乙酰基-2-甲基-5-对甲氧基苯基)吡咯,并通过红外光谱、核磁共振氢谱及元素分析对化合物进行了表征,并初步探讨了合成产物的影响条件。关键词 3-乙酰基-1-对甲氧基苯基-1,4-戊二酮,Paal-Knorr反应,二胺,吡咯 1 引言
吡咯及其衍生物是一类重要的杂环化合物,是许多天然化合物的重要组成部分并展示了良好的生理活性[1],在生物体的生长发育,能量储存和转化及生命信息传递等过程中具有重要作用[2],一直备受科学工作者的重视。近年来,吡咯及其衍生物已经被广泛用于制备药物、杀虫剂、合成材料[3]和有机光致变色材料,如吡咯类俘精酸酐[4]等。吡咯的合成一直是科学工作的热点之一,近年来,报道较多的是合成多取代的单吡咯化合物,而多取代的双吡咯化合物则报道较少[5]。
以3-乙酰基-1-对甲氧基苯基-1,4-戊二酮和不同的二胺为原料,通过 Paal-Knorr 反应,合成了系列N,N'-二取代(3-乙酰基-2-甲基-5-对甲氧基苯基)吡咯,并通过红外光谱、核磁共振氢谱及元素分析对化合物进行了表征。产品还未见报道。
2 实验
2.1 仪器和试剂
红外光谱用 Bruker VECTOR 22 红外分光光度计(KBr);核磁共振氢谱用 Bruker ANAVCE-400 型超导核磁共振仪(TMS 为内标,CDCl3 为溶剂);元素分析用PE-2400 C.H.N.元素分析仪;熔点测定用XT-4型双目显微熔点测定仪(温度计未校正)。
所有试剂和溶剂均为市售分析纯,其中无水乙醇用金属钠处理。w-溴-p -甲氧基苯乙酮按文献制备[6]。
2.2 3-乙酰基-1-对甲氧基苯基-1,4-戊二酮的合成
在装有冷凝管、恒压漏斗的三颈瓶中加入300 mL无水乙醇,搅拌下加入 11.6 g (0.5 mol) 钠,待钠全部溶解后,在搅拌下滴加50.5 g (0.5 mol) 乙酰丙酮,滴加完毕后充分搅拌 2h。控速滴加107.8 g(0.47 mol)w-溴-p -甲氧基苯乙酮的丙酮(300ml)溶液,TLC 跟踪 (Scheme 1)。反应物消失后将溶液倒入冰水中,抽滤得粗产品,乙醇-石油醚重结晶,得到白色晶体 1。
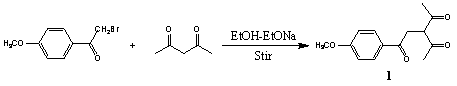
Scheme 1
化合物
1:yield 77.4%, m.p. 62.5-63℃. 1H NMR (CDCl3, 400 MHz) d: 2.37 (s, 6H, 2COCH3), 3.57 (d, J = 6.8 Hz, 2H, CH2), d: 3.90 (s, 3H, OCH3), 4.40 (t, J = 6.8 Hz, 1H, CH), 6.96 (d, J = 8.8 Hz, 2H, 2Ar-H), 7.97 (d, J = 8.8 Hz, 2H, 2Ar-H), IR (KBr) ν: 1710, 1670, 1430, 1365, 1360, 1040 cm-1. Anal. calcd for C14H16O4: C 67.74, H 6.45; found: C 67.73, H 6.42.2.3 N,N′-二取代(3-乙酰基-2-甲基-5-对甲氧基苯基)吡咯的合成
在装有冷凝管的圆底烧瓶中加入化合物 1 0.5 g (2.0 mmol) 、二胺 2 a-2h (1.0 mmol)、乙酸和无水乙醇混合液 (v/v: 3:4, 14ml),搅拌回流,TLC 跟踪 (Scheme 2) 。反应完毕后冷却,减压蒸去溶剂得粗产品,氯仿和无水乙醇重结晶得纯产品 3a-3h。
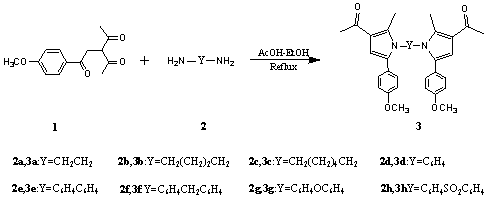
化合物
3a-3h 的产率、熔点及表征数据如下:化合物3a: yield 84.4%, m.p. 199-200℃. 1H NMR (CDCl3, 400 MHz) d: 2.07 (s, 6H, 2CH3), 2.40 (s, 6H, 2COCH3), 3.87 (s, 4H, 2CH2), 3.88 (s, 6H, 2OCH3), 6.37 (s, 2H, 2×Py-H), 6.92 (d, J = 8.8 Hz, 4H, 2×2Ar-H), 7.07 (d, J = 8.8 Hz, 4H, 2×2Ar-H). IR (KBr) n: 1655, 1355, 1350, 1035 cm-1. Anal. calcd for C30H32N2O4: C 74.36, H 6.66, N 5.78; found: C 74.37, H 6.62, N 5.75.
化合物3b: yield 77.8%, m.p. 148-149℃. 1H NMR (CDCl3, 400 MHz) d: 2.43 (s, 6H, 2CH3), 2.53 (s, 6H, 2COCH3), 3.89 (s, 6H, 2OCH3), 1.29 (s, 4H, 2×CH2), 3.70 (s, 4H, 2×CH2N), 6.41 (s, 2H, 2×Py-H), 6.93 (d, J = 8.4 Hz, 4H, 2×2Ar-H), 7.14 (d, J = 8.4 Hz, 4H, 2×2Ar-H). IR (KBr) n: 1655, 1490, 1365, 1030 cm-1. Anal. calcd for C32H36N2O4: C 74.98, H 7.08, N 5.46; found: C 74.95, H 7.09, N 5.43.
化合物3c: yield 77.1%, m.p. 142.5-143℃. 1H NMR (CDCl3, 400 MHz) d: 2.43 (s, 6H, 2CH3), 2.60 (s, 6H, 2COCH3), 3.87 (s, 6H, 2OCH3), 1.01 (s, 4H, 2×CH2), 1.43 (s, 4H, 2×CH2), 3.78 (t, J = 7.6 Hz, 4H, 2×CH2N), 6.42 (s, 2H, 2×Py-H), 6.95 (d, J = 8.4 Hz, 4H, 2×2Ar-H), 7.24 (d, J = 8.8 Hz, 4H, 2×2Ar-H). IR (KBr) n: 1660, 1415, 1355, 1040 cm-1. Anal. calcd for C34H40N2O4: C 75.53, H 7.46, N 5.18; found: C 75.51, H 7.45, N 5.16.
化合物3d: yield 81.9%, m.p. 286-287℃. 1H NMR (CDCl3, 400 MHz) d: 2.45 (s, 6H, 2CH3), 2.52 (s, 6H, 2COCH3), 3.81 (s, 6H, 2OCH3), 6.67 (s, 2H, 2×Py-H), 6.75 (d, J = 8.4 Hz, 4H, 2×2Ar-H), 7.00 (d, J = 8.8 Hz, 4H, 2×2Ar-H), 7.19 (s, 4H, 4×Ar-H). IR (KBr) n: 1645, 1400, 1355, 1035 cm-1. Anal. calcd for C34H32N2O4: C 76.67, H 6.06, N 5.26; found: C 76.68, H 6.04, N 5.27.
化合物3e: yield 79.0%, m.p. 298-299℃. 1H NMR (CDCl3, 400 MHz) d: 2.49 (s, 6H, 2CH3), 2.53 (s, 6H, 2COCH3), 3.78 (s, 6H, 2OCH3), 6.69 (s, 2H, 2×Py-H), 6.76 (d, J = 8.4 Hz, 4H, 2×2Ar-H), 7.05 (d, J = 8.4 Hz, 4H, 2×2Ar-H), 7.24 (d, J = 8.0 Hz, 4H, 2×2Ar-H), 7.66 (d, J = 8.0 Hz, 4H, 2×2Ar-H). IR (KBr) n: 1645, 1490, 1355, 1040 cm-1. Anal. calcd for C40H36N2O4: C 78.93, H 5.96, N 4.60; found: C 74.95, H 5.98, N 4.59.
化合物3f: yield 83.0%, m.p. 181-181.5℃. 1H NMR (CDCl3, 400 MHz) d: 2.45 (s, 6H, 2CH3), 2.52 (s, 6H, 2COCH3), 3.76 (s, 6H, 2OCH3), 4.08 (s, 2H, CH2), 6.66 (s, 2H, 2×Py-H), 6.71 (d, J = 8.8 Hz, 4H, 2×2Ar-H), 7.00 (d, J = 8.8 Hz, 4H, 2×2Ar-H), 7.09 (d, J = 8.0 Hz, 4H, 2×2Ar-H), 7.20 (d, J = 8.4 Hz, 4H, 2×2Ar-H). IR (KBr) n: 1650, 1495, 1410, 1355, 1040 cm-1. Anal. calcd for C41H38N2O4: C 79.08, H 6.15, N 4.50; found: C 79.10, H 6.13, N 4.51.
化合物3g: yield 72.0%, m.p. 114-116℃. 1H NMR (CDCl3, 400 MHz) d: 2.47 (s, 6H, 2CH3), 2.52 (s, 6H, 2COCH3), 3.79 (s, 6H, 2OCH3), 6.67 (s, 2H, 2×Py-H), 6.76 (d, J = 8.4 Hz, 4H, 2×2Ar-H), 7.03 (d, 4H, J = 8.4 Hz, 2×2Ar-H), 7.07 (d, J = 8.4 Hz, 4H, 2×2Ar-H), 7.14 (d, J = 8.4 Hz, 4H, 2×2Ar-H). IR (KBr) n: 1655, 1500, 1405, 1235, 1035 cm-1. Anal. calcd for C40H36N2O5: C 76.90, H 5.81, N 4.48; found: C 76.92, H 5.80, N 4.45.
化合物3h: yield 62.7%, m.p. 233-235℃. 1H NMR (CDCl3, 400 MHz) d: 2.44 (s, 6H, 2CH3), 2.51 (s, 6H, 2COCH3), 3.76 (s, 6H, 2OCH3), 6.67 (s, 2H, 2×Py-H), 6.70 (d, J = 8.4 Hz, 4H, 2×2Ar-H), 6.91 (d, J = 8.4 Hz, 4H, 2×2Ar-H), 7.31 (d, J = 8.4 Hz, 4H, 2×2Ar-H), 7.99 (d, J = 8.8 Hz, 4H, 2×2Ar-H). IR (KBr) n: 1660, 1340, 1335, 1165, 1040 cm-1. Anal. calcd for C40H36N2SO6: C 71.41, H 5.39, N 4.16; found: C 71.43, H 5.40, N 4.18.
3 结果和讨论
3.1 反应物的物质的量比对产物的影响
按照物质的量的比为 2:1,在乙酸和乙醇的混合液中回流合成了化合物3a-3h。为了研究原料的比例对产物的影响,我们进行了对比实验。
在相同条件下,0.5g (2.0mmol) 1 and 0.2g (2.0 mmol) 2d 混合回流,反应结束后柱色谱分离,石油醚和乙酸乙脂(v/v=2:3)做洗脱剂得到化合物3d(0.36g, Yield 67.2%), 随后用乙酸乙酯做洗脱剂得到化合物N-对乙酰氨基苯基-3-乙酰基-2-甲基-5-对甲氧基苯基吡咯 4(0.12g, Yield 16.9%) (Scheme 3)。而用 0.5g (2.0mmol) 1 and 0.1g (1.0 mmol) 2d 反应,也用柱色谱分离只得到了化合物 3d。
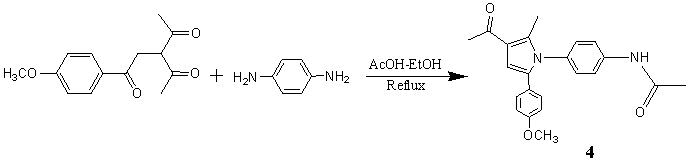
Scheme 3
化合物 4:m.p.199-201. 1H NMR (CDCl3,
400 MHz) d: 2.22 ( s, 3H, CH3
), 2.42 (s, 3H, COCH3), 2.51 (s, 3H, COCH3), 3.77 (s, 3H, OCH3),
6.65 (s, H, Py-H), 6.73 (d, J = 8.4 Hz, 2H, 2Ar-H), 7.01 (d, J = 8.8 Hz, 2H,
2Ar-H), 7.09 (d, J = 8.4 Hz, 2H, 2Ar-H), 7.53 (s, H, NH), 7.59 (d, J = 8.4
Hz, 2H, 2Ar-H). IR (KBr) n: 3270, 1655, 1530, 1415, 1325, 1320,
1035 cm-1. Anal. calcd for C22H22N2O3:
C 72.91, H 6.12, N 7.73; found: C 72.94, H 6.11, N 7.76.
以上实验结果说明:不同比例的原料混合回流,多取代的双吡咯是主要产物。同时推测其它的二胺将出现相似的结果。
3.2 反应物的滴加顺序对产物的影响
为研究能否获得较多的单吡咯,我们改变了原料的加入顺序。在装有冷凝管和恒压漏斗的烧瓶中加入化合物
2d 0.2g (2.0 mmol) 、冰乙酸和无水乙醇混合液 (v/v: 3:4, 7ml),在回流状态下滴加化合物 1(0.5 g、2.0 mmol)的冰乙酸和无水乙醇 (v/v: 3:4, 7ml)溶液。反应完毕后柱色谱分离得到3d(0.11g,
Yield 20.5%) and 4(0.42g, Yield 59.2%)。可以发现化合物
4 的产率有所提高。
4 结论
在本文中,我们合成了八种多取代的双吡咯化合物并通过 IR, 1H NMR 和元素分析对产物做了表征,同时研究了1,4-二酮和二胺合成单吡咯的反应条件。
REFERENCES
[1] (a) Hua W T. Heterocyclic Chemistry (Zahuan Huaxue). Beijing: Perking
University Publishing Corporation, 1991. (b) Joule J. A., Mills A.. Heterocyclic Chemistry
(Zahuan Huaxue ). Fourth edition. Beijing: Science Press , 2004.
[2] Zen X C, Xu S H, Li Y Q et al.. Chin. J. Org. Chem. (Youji Huaxue), 2005, 25 (11):
1420.
[3] (a) Furstner A, Weintritt H, Hupperts A. J. Org. Chem., 1995, 60 (20): 6637; (b)
Khanna I. K., Weier R. M., Yu Y et al. J. Med. Chem , 1997, 40 (11): 1619; (c)
Thurkauf A., Yuan J., Chen X et al. J. Med. Chem., 1995, 38 (25): 4950.
[4] (a) Seibold M., Port H., Gustav K.. Chem. Phys. Lett. (1-2), 1999, 314: 65; (b) Wang Y
L, Yao B L, Lei M et al. Acta Optica Sinica (Guangxue Xuebao), 2003, 23 (5): 616; (c)
Menke N M L, Yao B L, Wang Y L, et al.. Acta Photonica Sinica (Guangzi Xuebao), 2004, 33
(5): 581.
[5] (a) Fan G B, Ming Y F, Fan M G. Science in China(Series B) (Zhongguo Kexue B), 1995,
25 (12): 1257; (b) Zhang S W, Cheng Z H, Li Z Y et al. CAIJ, 2005, 2 (7): 225; (c) Cheng Z
H, Zhang S W, Li Z Y et al. Chin.J.Org.Chem (Youji Huaxue), 2006, 26(1): 90.
[6] Su B, Bao Y J, Li H J et al.. Chin. J. Pharmaceuticals.(Zhongguo Yiyao Gongye Zazhi),
2001, 32 (8): 374.