http://www.chemistrymag.org/cji/2007/096029ne.htm |
Jun.10,
2007 Vol.9 No.6 P.29 Copyright |
CaoYuqing, Guo Yanxin , Li Yabin
(College of Pharmacy, Hebei University, Baoding 071002, China)
Received on June. 7, 2007.
Abstract 3,4-Dihydropyrimidin-2(1H)-one
and their derivatives were synthesized in good yields (71-93%) by one-pot three-component
Biginelli condensation in the presence of KH2PO4 and PEG400 as
catalyst under solvent-free conditions. And the reaction time is shortened from 18hr to
1.5-6.5hr. This approach of direct reaction without solvent shows a new direction in green
synthesis.
Keywords Biginelli Reaction, PEG400 - KH2PO4, Solvent-Free
1. INTRODUCTION
The toxicity and volatile nature of many organic solvents, particularly chlorinated
hydrocarbons, that are widely used in large amounts for organic reactions have
posed as serious threat to the environment [1]. Thus, the design of solventless
catalytic reaction has received tremendous attention in recent times in the area of green
synthesis [2].
Dihydropyrimidinones (DHPMs) and their derivatives occupy an important
place in the realm of natural and synthetic organic chemistry because of their therapeutic
and pharmacological properties [3]. They have emerged as integral backbones of
several calcium channel blockers, anti-hypertensive agents, R-1a-antagonists and
neuropeptide Y (NPY) antagonists [4]. Moreover, several alkaloids containing
the dihydropyrimidine core units have been isolated from marine sources, which also
exhibit interesting biological properties [5]. Most notable among these are the
batzelladine alkaloids, which were found to be potent HIVgp-120-CD4 inhibitors [6].
Thus, the synthesis of this heterocyclic nucleus is of much current importance.
Biginelli reaction, first reported in 1893 [7], is a one-pot
three-component condensation using b-dicarbonyl compounds with aldehydes and urea or thiourea
derivatives in the presence of acid in ethanol furnishing 3,4-Dihydropyrimidin-2(1H)-ones.
This reaction is carried out by simply heating a mixture of the above three components
dissolved in ethanol with catalytic amount of hydrochloric acid at reflux temperature for
18 hours. However, this one-pot, one step protocol provides only low to moderate yields of
the desired product (20-50%), in particular when substituted aromatic or aliphatic
aldehydes are employed. Therefore, the searching of practical methods for the synthesis of
dihydropyrimidin-2 (1H)-ones by the Biginelli reaction continues to attract the attention
of researchers. Several improved procedures for the preparation of DHPMs have recently
been reported based on Lewis acids [8], solid support [9] or
microwave [10] or on reagents like Mn (OAc) 3[11], I2
[12], LiBr [13], KHSO4 [14]. Recently, a
number of procedures under solvent-free conditions using Yb (OTf) 3[15],
montmorillonite [16] and ionic liquid [17] as catalysts have been
reported. Obviously, many of these methods involve expensive reagents; stoichiometric
amounts of catalysts; strongly acidic conditions; long reaction time, unsatisfactory
yields and incompatibility with other functional group and the solvent used are not at all
acceptable in the context of green synthesis. On the other hand, an inevitable problem
exists when Lewis acid used as catalyst, that is a reaction of Lewis acid
with water. Therefore, seeking for a new and an inexpensive catalyst for the preparation
of dihydropyrimidinones under mild conditions is of prime importance.
Polyethylene glycols (PEGs) have been widely used as PTC in many
organic reactions owing to their stability, low cost, environment-friendly, and easy
availability. It has been proved that PEGs incorporating 7-9 units are more
effective in catalyzing the reactions in which K+ or Na+ salts participate. PEG400-600
was more suitable for liquid-liquid or solid-liquid phase solvent-free organic reactions.
In the continuation of our investigation on the research of using PEGs as PTC [18],
herein, we report a new study on the Biginelli condensation using KH2PO4
and PEG400 as catalysts under solvent-free conditions (Scheme. 1).
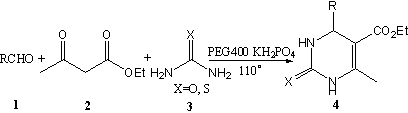
Scheme 1
The reaction conditions were studied by carrying out the condensation of benzaldehyde, ethyl acetoacetate, and urea as the reactants. The desired dihydropyrimidinine was obtained with good yield in the presence of a catalytic amount of KH2PO4 at 110oC. We also examined this reaction in different solvents, but the reaction gave better yield under solvent-free conditions than other tested (such as toluene (71.6%), dichloromethane (38.4%), tetrahydrofuran (62%), acetonitrile (45.8%). Thus, condensation of a mixture of benzaldehyde, ethyl acetoacetate and urea at 110oC in the presence of 10mol% of KH2PO4 furnished the corresponding dihydropyrimidinone product in 84% yield. The procedure gives the product in good yields and avoids the problems associated with solvent use (cost, handing, safety and pollution). The other reaction product, water, evaporates at the reaction temperature of 110oC.The efficacy of this procedure was equally high for aromatic aldehyde containing electron withdrawing and electron donating groups. The results are summarized in Table 1. Table 1. Synthesis of dihydropyrimidinones under solvent-free conditions.a
Entry |
R |
X |
Time(h) |
Yield (%)b |
M.p |
M.p (Lit*) |
4a |
C6H5 |
O |
1.5 |
84 |
204-207 |
202-203[14] |
|
|
O |
2.5 |
86c |
204-207 |
202-203[14] |
4b |
4-OHC6H4 |
O |
3 |
90 |
228-231 |
227-229[14] |
4c |
2-OHC6H4 |
O |
3.5 |
83 |
201-203 |
202-203[14] |
4d |
2-NO2C6H4 |
O |
4.5 |
85 |
207-209 |
208-210[11] |
4e |
4-NO2C6H4 |
O |
4 |
88 |
207-208 |
207-208[14] |
4f |
4-OCH3C6H4 |
O |
1.5 |
90 |
207-209 |
205-207[9] |
4g |
4-ClC6H4 |
O |
4 |
87 |
214-216 |
214-215[14] |
4h |
4-NMe2C6H4 |
O |
4.5 |
84 |
251-252 |
257-258[14] |
4i |
2-ClC6H4 |
O |
4.5 |
82 |
215-217 |
215-217[14] |
4j |
3-OHC6H4 |
O |
3 |
85 |
166-168 |
167-170[9] |
4k |
2-OCH3C6H4 |
O |
2 |
88 |
258-259 |
259-260[9] |
4l |
C6H5-CH=CH |
O |
3 |
84 |
231-233 |
230-232[9] |
4m |
3-NO2C6H4 |
O |
4.5 |
81 |
225-228 |
227-229[11] |
4n |
4-OH-3-OMeC6H3 |
O |
1.5 |
85 |
234-237 |
|
4o |
3,4-(OMe)2C6H3 |
O |
1.5 |
93 |
175-177 |
175-177[11] |
4p |
4-FC6H4 |
O |
4 |
84 |
182-183 |
182-184[9] |
4q |
n-C3H7 |
O |
6.5 |
71e |
155-157 |
154[9] |
4r |
(CH3)2CH2 |
O |
6 |
75e |
174-176 |
172-174[14] |
4s |
C6H5 |
S |
3.5 |
82 |
205-207 |
209-211[9] |
4s’ |
|
S |
7 |
78d |
205-207 |
209-211[9] |
4t |
3-OHC6H4 |
S |
4.5 |
80 |
183-184 |
183-184[9] |
4u |
3-NO2C6H4 |
S |
4.5 |
81 |
202-204 |
203-205[9] |
4v |
4-OMeC6H4 |
S |
3 |
85 |
152-154 |
153-155[9] |
4w |
4-OHC6H4 |
S |
4.5 |
89 |
195-197 |
b Isolated yields. c Without PEG400. d No catalyst and no solvent. e reaction temp 70oC. From the data in Table 1, the condensation of a series of aldehydes with ethyl acetoacetate and urea or thiourea leading to 3,4-Dihydropyrimidin-2 (1H)-ones give good yield under solvent-free conditions than that of the classical method. For example, products 4b, 4e, 4f were obtained in 90%, 88%, 89% yields, whereas, in the classical method [7], the yields of 4b, 4e, 4f were 67%, 58%, 61% respectively in 18hr. The reaction was examined without any catalyst and solvent. Based on the results of the comparative experiments (Entry4s, Entry4s'), we suggest that the catalyst is important for the reaction. Aromatic aldehydes which carrying either electron-withdrawing or electron-donating substituents obtained good yields of products. This method is effective even with a,b-unsaturated aldehydes which is usually produced with poor yields in the presence of protic or Lewis acids due to their decomposition or polymerization under acidic conditions. From the reaction times in Table 1, it has been found that the reaction of aldehydes bearing -NO2 groups on the aryl ring can be carried out in relatively longer time than that of the aldehydes bearing -OCH3 on the aryl ring. The probable reason is that the electron-donating groups increase the activity of carbonyl group in the benzaldehydes, while the electron-withdraw ing groups decrease the activity. O-substituted aromatic aldehydes needed relatively long reaction times, but low yields were found because of the influence of steric hindrance. Another important feature of this procedure is its efficiency for the good yield synthesis of DHPMS from aliphatic aldehydes.
Thiourea has been used with similar success to provide the corresponding thio-derivatives of dihydropyrimidinones that are also of much interest with respect to their biological activities. Thus, each of benzaldehyde (Entry4s), p-methoxy (Entry4v) and p-hydroxy (Entry4w) reacted efficiently with ethyl acetoacetate and thiourea affording the corresponding S-HPMS in good yields. However, from the data in Table 1, it can be seen that a longer reaction time was necessary for the thiourea to obtain good yield.
In summary, a novel method for Biginelli condensation catalyzed by PEG400 and anhydrous KH2PO4 without solvent was developed. The advantages of this method were safety, high selectivity, environment friendly and ease of work-up.
3. EXPERIMENTAL SECTION
1H-NMR spectra were obtained on a Bruker AVANCE (400MHz) spectrometer using
TMS as internal standard and DMSO as solvent. TLC was GF254 thin layer chromatography with
petroleum ether/ethyl acetate as eluent. aldehydes, ethyl acetoacetate, urea and thiourea
were all commercial products and were used without further purification. All liquid
reagents were distilled before use. Melting points were determined on a microscopy
apparatus and are uncorrected.
Typical experimental procedure for the preparation of compounds (4a-4w):
A
mixture of aromatic aldehydes (1, 0.1mol), ethyl aectoacetate (2, 0.1mol),
urea or thiourea (3, 0.1mol), KH2PO4 (1.36g), and PEG400
4e. 5-Ethoxycarbonyl-4- (4-nitroxyphenyl)-6-methyl-3, 4-dihydropyrimidin-2 (1H)-2-one
White crystals (EtOH) 1H-NMR (DMSO- d6): d =9.36(s, 1H, NH), 7.85 (s, 1H, NH), 8.22-7.45 (m, 4H, Ar), 5.26 (s, 1H, CH), 3.97 (q, J=7.0Hz, 2H, OCH2CH3), 2.25 (s, 3H, CH3), 1.08 (t, J=7.0Hz, 3H, -OCH2CH3).
4m. 5-Ethoxycarbonyl-4-(3-nitroxyphenyl)-6-methyl-3, 4-dihydropyrimidin-2 (1H)-2-one
White crystals (EtOH) 1H-NMR (DMSO-d6): d =9.37 (s, 1H, NH), 7.90 (s, 1H, NH)), 8.1-7.63 (m, 1H, Ar), 5.29 (s, 1H, CH) 3.98 (q, J=6.9Hz, 2H, -OCH2CH3), 2.26 (s, 3H, CH3), 1.08 (t, J=6.9Hz, 3H, -OCH2CH3).
4n.5-Ethoxycarbonyl-4-(4-hydroxy-3-methoxyphenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-2 one
White crystals (EtOH) 1H-NMR (DMSO-d6): d =9.15 (s, 1H,), 8.93 ( s, 1H,), 7.66 ( s, 1H), 6.80 ( s, 1H), 6.74 (d, J=8.4Hz, 1H), 6.61 (d, J=8.4Hz, 1H), 5.05 (d, J=2.8Hz, 1H), 3.98 (q, J=7.2Hz, 2H, -OCH2CH3), 3.72 (s, 3H, CH3), 2.21 (s, 3H, CH3), 1.11 (t, J=7.2Hz, 3H, -OCH2CH3).
4w. 5-Ethoxycarbonyl-4-(4-hydroxyphenyl)-6-methyl-3, 4-dihydropyrimidin-2 (1H)-2-thione
White crystals (EtOH) 1H-NMR (DMSO-d6): d =9.37 (s, 1H, OH), 10.50 (s,1H, NH), 9.67 (s, 1H, NH), 8.22-7.54 (m, 4H, Ar), 5.27 (s, 1H, CH), 4.00 (q, J=7.1Hz, 2H, -OCH2CH3), 2.34 (s, 3H, CH3), 1.24 (t, J=7.1Hz, 3H, -OCH2CH3).
REFERENCES
[1 ] Nelson W M, Anastas P T, Williamson T C, Ed: Oxford University Press: Oxford,
1998, Chapter, 12, 200.
[2] (a) Tanaka K, Toda F Chem. Rev, 2000, 100:1025-1074; (b) Cave G W V, Raston C L, Scott
J L. Chem. Commun, 2001, 21: 2159.
[3] Kappe C O. Tetrahedron, 1993, 49: 6937.
[4] (a) Atwal K S, Rovnyak G C, Kimball S D, et al. J. Med. Chem, 1990, 33: 2629-2635; (b)
Rovnyak G C, Kimball S D, Beyer B, et al. J. Med. Chem, 1995, 38: 119.
[5] Overman M H, Rabinowitz A P. J. Am. Chem. Soc, 1995, 117: 2657.
[6] Patil A D, Kumar N V, Kokke W C, et al. J. Org. Chem, 1995, 60: 1182.
[7] Biginelli P, Chim G. Ital, 1893, 23: 360.
[8] (a) Ranu B C, Hajra A, Jana U. J. Org. Chem, 2000, 65: 6270; (b) Reddy Ch V, Mahesh M,
Raju P V K, et al. Tetrahedron. Lett, 2002, 43: 2657-2659; (c) Wang Z, Xu L, Xia
Ch, et al. Tetrahedtron. Lett, 2004, 45: 7951; (d) Lu J, Bai Y, Wang Z, et al.
Tetrahedron. Lett, 2000, 41: 9075-9078; (e) Surya K, D R, Gibbs A. Synthesis, 2005,
11: 1748.
[9] Salehi P, Dabiri M, Zolfigol M A, et al. Tetrahedron. Lett, 2003, 44: 2889.
[10] Kidwai M, Saxena S, Mohan R, et al. J.
Chem. Soc. Perkin Trans, 2002, 1: 1845.
[11] Kumar K A, Kasthuraiah M, Suresh R C, et al.
Tetrahedron Lett, 2001, 42: 7873.
[12] Bhosale R S, Bhosale S V, Bhosale S V, et al. Tetrahedron Lett, 2004, 45: 9111.
[13] Maiti G, Kundu P, Guin C. Tetrahedron. Lett, 2003, 44: 2757
[14] Tu S J, Fang F, Zhu S L, et al. Synlett, 2004, 3: 537.
[15] Ma Y, Qian C, Wang L, et al. J. Org. Chem, 2000, 65: 3864.
[16] Lin H, Ding J, Chen X, et al. Molecules, 2000, 5: 1240.
[17] Peng J, Deng Y. Tetrahedron Lett, 2001, 42: 5917.
[18] Cao Y Q, Dai Z, Zhang R. Synth. Commun, 2005, 35: 1045.
曹玉庆, 郭艳欣, 李亚彬
(河北大学药学院, 保定, 071002 )
摘要 无溶剂条件下,PEG400和KH2PO4作催化剂, 一锅法三组分的Biginelli缩合反应合成3,4-二氢嘧啶-2(1H)-酮及其衍生物,收率良好(71-93%)。 反应时间由18个小时缩短到1.5-6.5个小时。此方法直接, 简便, 无溶剂, 展现了绿色合成的一个新方向。
关键词 Beginelli反应,PEG400 - KH2PO4,无溶剂