http://www.chemistrymag.org/cji/2007/097033pe.htm |
Jul.12,
2007 Vol.9 No.7 P.33 Copyright |
Study on the solid phase extraction and spectrophotometric determination of mercury in tobacco and tobacco additives with 5-(p-aminobenzylidene)-thiothiorhodanine
Hu Qun 1, Liu Zhihua 1,
Wang Jian 1, Hu Lin 2
(1 Yunnan Academy of Tobacco Science, Kunming, 650223, China; 2
Life science school, Yunnan University, Kunming 650091, China Yunnan Universty, Kunming,650091, China)
Abstract A high sensitive, selective and rapid
method for the determination of mercury based on the rapid reaction of mercury(II) with
5-(p-aminobenzylidene)-thiothiorhodanine (ABTR) and the solid phase
extraction of the colored chelate with C18 disks has been developed. In the
presence of pH = 3.5 sodium acetate-acetic acid buffer solution and Emulsifier-OP medium,
ABTR reacts with mercury(II) to form a red chelate of a molar ratio 1:2 (mercury to ABTR).
This chelate was enriched by the solid phase extraction with C18 disks and
eluted the retained chelate from the disks with dimethyl formamide (DMF). The enrichment
factor of 50 was achieved. In the DMF medium, the molar absorptivity of the chelate is
1.21×105 L.mol-1.cm-1
at 545 nm. Bee's law is obeyed in the range of 0.01~ 3 m g/ml in the measured
solution. The relative standard deviation for eleven replicates sample of 0.1 m g/ml level
is 1.69%. This method was applied to the determination of mercury in tobacco and tobacco
additives with good results.
Keywords mercury, solid phase extraction, spectrophotometry,
5-(p-aminobenzylidene)-thiothiorhodanine
1 INTRODUCTION
Mercury is a toxic heavy metal ion. Determination of trace mercury in tobacco and tobacco
additive is very important. The Quality Standards of Tobacco in Chinese Tobacco Company
defines that the concentration of mercury should not exceed 0.2 m g/g in tobacco additive
[1]. Many sensitive instruments, such as spectrofluorimetry, X-ray fluorescence
spectrometry, neutron activation analysis, atomic absorption spectrometry,
chemiluminescence, electrochemical analysis, and the like have widely been applied to the
determination of mercury [2-8]. But the spectrophotometric method still has the
advantages of its simplicity and no need of expensive or complicated test equipment. For
this reasons, a wide variety of spectrophotometric methods for the determination of
mercury have been reported [9-19]. Each chromogenic system has its advantages
and disadvantages with respect to sensitivity, selectivity and rapidity.
However, for the routine spectrophotometric methods determination low
concentration of mercury ion, a preconcentration step is usually required. Solid phase
extraction is an attractive technique because of its notable advantages [20-24].
In this work, a new chromogenic reagent 5-(p-aminobenzylidene)-thiothiorhodanine (ABTR)
was synthesized. The color reaction of SBDTR with mercury and the solid phase extraction
of the colored chelate with C18 disks were studied. On this basis, a highly
sensitive, selective and rapid method for the determination of mercury in tobacco and
tobacco additives was developed.
2.1 Apparatus
A UV-2401 spectrophotometer (Shimidzu, Japanese), equipped with a 1 cm microcell (0.5 ml) was used for all absorbance measurements. The pH measurements were made with a Beckman F-200 pH meter (Beckman Instruments, USA). The extraction was performed on Waters Solid Phase Extraction (SPE) Device (able to prepare twenty samples simultaneously), and Zorbax C18 membrane disks [47 mm (diameter)× 0.5 mm (thickness), 8 m m, 50 mg] (Agilent Technologies, USA) was used.
2.2 Reagents
The ABTR was synthesized as the following procedure: 40 ml of acetic acid was added to the sample of 1.5 g of thiorhodanine and 1.2 grams of p-aminobenzaldehyde, and the mixture was heated gently to dissolve the thiorhodanine and p-aminobenzaldehyde completely. The solution was refluxed for about 1.5 h, and about 1 ml of concentrated sulfuric acid was added dropwise. After the color of the solution turned red, the refluxing was stopped and the sample was poured into 200 ml of distilled water. After the red solids precipitated from the solution, a small amount of aqueous ammonia was added. Then the precipitants were separated by filtration. The precipitants were recrystallized twice from absolute alcohol and the yield was 36% (m. p. 282ºC~285ºC). The structure has been verified as shown in Fig.1 by elemental analysis, IR, 1HNMR and MS. Elemental analysis results were shown as follows: calculated: 47.59% C, 3.19% H, 11.10% N, 38.12% S; found: 47.23% C, 3.28% H, 11.02% N, 37.53% S. IR (KBr) (cm-1): 3470, 3450, 3355 (nN-H); 3060, 3020 (n=C-H); 1628 (dN-H); 1566, 1548, 1515, 1450 (nC=C); 1292 (nC-N); 1171 (nC=S); 825 (dAr-H); 806 (dC=C-H). 1HNMR (solvent: DMSO-d6) (d,ppm): 7.46 (1H, s, C=C-H); 7.26, 7.35 (2H, d, J≈9Hz, H-2 and H-6); 6.62, 6.72 (2H, d, J≈9Hz, H-3 and H-5); 3.36 (2H, w, -NH2). MS (EI) (m/z): 252 (M+·).
All of the solutions were prepared with ultra-pure water obtained from a Milli-Q50 SP Reagent Water System (Millipore Corporation, USA). High purity dimethyl formamide (DMF) (Fisher Corporation, USA) was used. A 3.0×10-4 mol/L of ABTR solution was prepared by dissolving ABTR with ethanol. A stock standard solution of mercury (1.0 mg/ml) was obtained from Chinese Material Standard Center, and a work solution (0.5 m g/ml) was prepared by diluting this solution. 0.5 mol/L of pH = 3.5 sodium acetate-acetic acid buffer solution(containing 0.2 mol/L of pyrophosphoric acid). Emulsifier-OP solution (2.0 % (v/v)) was prepared by dissolving emulsifier-OP with water. All chemicals used were of analytical grade unless otherwise stated.
Fig.1 The structure of ABTR
2.3 General Procedure
To a standard or sample solution containing no more than 3.0 m g of Hg(II) in a 50 ml
of calibrated flask, 5 ml of 0.5 mol/L sodium acetate-acetic acid (containing 0.2 mol/L pyrophosphoric acid)with pH 3.5, 3.0 ml of 3.0×10-4 mol/L ABTR
solution and 2.0 ml of 2.0 % Emulsifier-OP solution were added. The mixture was diluted to
volume of 50 ml and mixed well. After 10 min, the solution was passed through the C18
disks at a flow rate of 50 ml/min. the colored chelate would be retained on the disks.
After the enrichment had finished, eluted the retained chelate from the disks at a flow
rate of 5 ml/min in reverse direction with 1.0 ml of DMF. The absorbance of this solution
was measured at 545 nm in a 1cm microcell (0.5 ml) against a reagent blank prepared in a
similar way without mercury.
3 RESULTS AND DISCUSSION
3.1 Absorption Spectra
The absorption spectra of ABTR and its Hg(II) chelate are shown in Fig 2. The absorption peaks of ABTR and its complex in DMF medium are located at 420 nm and 545 nm.
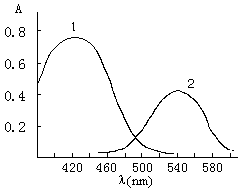
Fig. 2 Absorption spectra of ABTR and its Hg(II) complex: 1 ABTR-Emulsifier-OP blank against water; 2 ABTR-Emulsifier-OP-Hg(II) chelate against reagent blank.
3.2 Effect of acidity
Results showed that the optimal pH for the reaction of Hg(II)
with ABTR is 1.2- 4.2. A sodium acetate-acetic acid buffer solution of pH 3.5 was
recommended, as the use of 4~ 6 ml of the buffer solution (pH 3.5) per 50 ml of final
solution was found to give a maximum and constant absorbance. The use of 5 ml buffer
solution was recommended. The buffer solution containing a 0.15~ 0.25 mol/L of
pyrophosphoric acid can greatly increase the selectivity and do not affect the sensitivity
(Without pyrophosphoric in the buffer solution, the tolerance limits of foreign ions were
0.005 mg for Cu(II), Ag(I); 0.002 mg for Pd(II), Pb(II). However, the tolerance limits of
foreign ions reaches 0.5 mg for Cu(II); 0.2 mg For Pb(II); 0.1 mg for Pd(II), Ag(I) when
pyrophosphoric existed in the buffer solution). So 0.2 mol/L of pyrophosphoric acid in
buffer was recommended.
3.3 Effect of surfactants
The effects of surfactants on Hg(II)-ABTR system were studied. The results (Table 1) showed that absence of surfactants, anionic surfactants or cationic surfactants, the Hg(II)-ABTR chromogenic system give a low absorption, whereas in the presence of nonionic surfactants, the absorption of the chromogenic system increases markedly. Various nonionic surfactants enhance the absorbance in the following sequence: Emulsifier-OP >Tween-80 > Tween-20 > Tween-60. Accordingly, Emulsifier-OP was additive and the use of 0.5- 3 ml of Emulsifier-OP solution give a constant and maximum absorbance. The use of 2.0 ml was recommended.
Table 1 The Effect of Surfactants on Hg(II)-ABTR Chromogenic System
Surfactant |
Absence |
Emulsifier-OP |
Tween-80 |
Tween-20 |
Tween-60 |
SDS |
CTMAB |
CPB |
l max (nm) |
520 |
545 |
540 |
540 |
535 |
530 |
530 |
530 |
e (×104) |
6.67 |
12.1 |
11.2 |
9.86 |
8.76 |
5.92 |
6.33 |
6.76 |
3.4 Effect of ABTR Concentration
For up to 2.0 m g of Hg(II), the use of about 3 ml
of 3.0×10-4 mol/L of ABTR solution has been found to be sufficient for a
complete reaction. Accordingly, 3.0 ml of ABTR solution were added in all further
measurement.
3.5 Stability of the Chromogenic System
After mixing the components, the absorbance reaches its maximum within 5 min at room
temperature and remains stable for at least 8 h. After extracted into the DMF medium, the
chelate was stable at least 12 h.
3.6 Solid phase extraction
Both the enrichment and elution were carried out on a Waters SPE device (able to
prepare twenty samples simultaneously). The flow rate was set to 50 ml/min during
enrichment and 5 ml/min during elution.
Some experiments were carried out in order to investigate the retention
of ABTR and its Hg(II) chelate on the disks. It was found that the ABTR and its Hg(II)
chelate could be retained on disks quantitatively when they pass the disks as aqueous
solution. The capacity of the disks for ABTR was 30 mg and for its Hg(II) chelate was 25
mg in 50 ml solution. In this experiment, the disks has adequate capacity to enrich the
Hg(II)-ABTR chelate.
In order to choose a proper eluant for the retained ABTR and its Hg(II)
chelate. Various organic solvents were studied. The effects of various organic solvents
were in the following sequence: DMF > acetonitrile > acetone > ethanol >
methanol. So DMF was selected as eluant. Experiments showed that it was easier to elute
the retained ABTR and its Hg(II) chelate in reverse direction than in forward direction,
so it is necessary to reverse the disks during elution. 1.0 ml of eluant was sufficient to
elute the ABTR and its Hg(II) chelate from disks at a flow rate of 5 ml/min. The volume of
1.0 ml eluant was selected.
3.7 Calibration Curve and Sensitivity
The calibration curve showed that Beer's law is obeyed in the concentration range of
0.01- 3 m g Hg(II) per ml in the measured solution. The linear regression equation
obtained was: A =0.581 C (m g/ml) + 0.0126 (r=0.9994). The molar
absorptivity was calculated to be 1.21×105 l.mol-1.cm-1
at 545 nm. The relative standard deviation at a concentration level of 0.1 m g/ml of
Hg(II) (11 repeats determination) was 1.69 %.
3.8 Composition of the complex
The composition of the complex was determined by continuous
variation method (Fig.3) and molar ratio method (Fig.4). Both showed that the molar ratio
of Hg(II) to ABTR is 1:2.
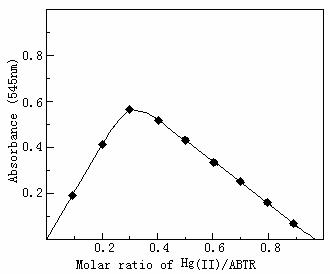
Fig.3 Composition of Hg(II)-ABTR complex by continuous variation method
The concentration of Hg(II)+ABTR was 1.2×105 mol.L-1, other conditions as in standard procedure
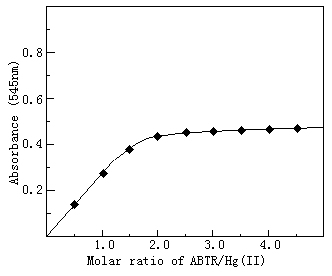
Fig.4 Composition of Hg(I1)-ABTR complex by molar ratio method
The concentration Hg(II) was 0.35×105 mol.L-1, other conditions as in standard procedure
3.9 Interference
The selectivity of the proposed method was investigated by the determination 1.0 m g/50ml
of Hg(II) in the presence of various ions within a relative error of ± 5% are given in
Table 2. The result showed most routine ions do not interfere with the determination. This
method is of high selectivity.
Table 2 Tolerance Limits for the Determination of 1.0 m g of Hg(II) with ABTR
(relative error ± 5% )
Ion added |
Tolerate(mg) |
NO3-, K+, borate, Na+ |
50 |
Li+, Al3+, PO43-, NO2-, SO42-, ClO4- |
20 |
Ca2+, Mg2+, SO32-, Sr2+, Ba2+, IO3-, BrO3-, ClO3- |
10 |
Mn2+, Ce(IV), W(VI), Mo(VI), U(IV), Fe3+ |
4 |
Ti(IV), Bi(III), V(V), Cr(VI), Zr(IV), F-, Fe2+, Cl- |
1 |
Cd2+, Cr3+, La3+, Sn(IV), Zn2+, Zr(IV) , Co2+, Ni2+, Cu2+ |
0.5 |
Ru(III), Bi(III), Pb2+, Sb3+, Th(IV), Br-, Os(VIII) , I- |
0.2 |
Se(IV), Te(IV), S2O32-, Pd+, Ag+ |
0.1 |
Ir(IV) , Rh(III), Ru(III) |
0.05 |
Pt(IV), Au3+ |
0.01 |
CN-, SCN- |
0.005 |
3.10 Application
The proposed method has been successfully applied to the
determination of mercury in tobacco and tobacco additive. 0.50 g of samples were weighed
accurately into the Teflon high-pressure microwave acid-digestion bomb (Fei Yue,
Analytical Instrument Factory, Shanghai, China). 3.0 ml of concentrated nitric acid and
5.0 ml of 30% hydrogen peroxide were added. The bombs were sealed tightly and then
positioned in the carousel of the microwave oven (Model WL 5001, 1000W, Fei Yue Analytical
Instrument Factory, Shanghai, China). The system was operated at full power for 8.0 min.
The digest was evaporated to near dryness. The residue was dissolved with 5 ml of 5% of
nitric acid, transferred into a 50 ml of calibrated flask quantitatively and analyzed by
general procedure. The recovery of mercury by added 0.2 m g of mercury in sample was
carried out, and a standard method using atomic absorption spectrometry was also used as
reference method. The results are shown in Table 3.
Samples |
Mercury found (mg/g) |
RSD% |
Recovery % |
|
This Method |
Reference Method |
|||
Tobacco leaf (SA1) |
0.165 |
0.152 |
2.4 |
96 |
Tobacco leaf (SA2) |
0.197 |
0.186 |
2.1 |
104 |
Glycerol (AL) |
0.228 |
0.212 |
2.3 |
103 |
Tobacco sauce (AM) |
0.157 |
0.162 |
2.2 |
105 |
Cigarette (SF) |
0.232 |
0.217 |
2.1 |
102 |
Tobacco essence (AG) |
0.185 |
0.176 |
2.5 |
97 |
Table 4 The comparison of reagents for the spectrophotometric determination of mercury
Reagents |
l max (nm) |
e(104 ) |
Linear range (mg/ml) |
Detect limit (ng/ml) |
Conditions |
Interference ions |
Ref |
H2Dz |
485 |
7.1 |
0.1~ 2 |
1 |
pH 2~ 5, extraction CCl3 |
Cu, Zn, Cd, Pb |
1 |
CPDAAB |
540 |
22.2 |
0.08- 0.8 |
20 |
alkaline medium, pH 10 |
Co,Zn, Cd, Ni |
11 |
DAA |
514 |
22 |
0~ 0.8 |
2 |
pH 9.0, Triton X-100 |
Cd, Ag, Pb, Ni |
13 |
BG |
625 |
59.6 |
0.004~ 0.5 |
- |
pH 5, flotation of Hg-I--BG with cyclohexane |
Ni |
14 |
CMPQ |
512 |
8.0 |
0.08~ 2 |
40 |
pH 4~ 4.8, Triton X-100 |
Co, Cu, Pb, Zn, Ni |
15 |
TPPS4 |
413 |
27.6 |
0~ 0.6 |
0.5 |
pH 8.0, heated in 100℃ water bath for 30nin |
Mn, Cu, Ag, Pb, Fe, Co, Ni, Zn, Cd, Mg |
16 |
PAR |
500 |
6.8 |
0.1~ 2 |
10 |
pH 10, |
Cu, Ni, Co, Fe, Zn, Ag, Cr, V, W, Mo, Mn |
17 |
NBS |
430 |
- |
1-10 |
0.2 |
nitric acid medium |
Cu, Zn, Cu |
18 |
ABTR |
545 |
12.1 |
0.01~ 3 |
0.02 |
pH 3.5, Emulsifier-OP |
CN-, SCN- |
This Work |
4 CONCLUSION
A comparison of the possibilities of the ABTR with those of other methods (Table 4) shows
that ABTR is a sensitive and selective spectrophotometric reagent for mercury. The molar
absorptivity of the chelate reaches 1.21×105
L.mol-1.cm-1.
When masked with pyrophosphoric acid, most of the routine foreign ions do not interfere
with the determination. This method has high selectivity. By solid phase extraction of
ABTR-Hg(II) chelate with C18 disks, the enrichment factor of 50 was achieved,
and the detection limit is 0.02 m g/L in the original samples. The consumption of organic
solvents in this method is much lower than those consumed in liquid-liquid extraction
method. By using Waters SPE device, twenty samples can be prepared simultaneously. This
method is rapid for simultaneously preparing large amount of samples. In a word, for the
determination of mercury, this method is high sensitivity, selectivity and rapidity.
REFERENCES
[1] National Standard of Chinese Tobacco Company, Tobacco and Tobacco additives,
YB850-83, Chinese Standards Press, 1985
[2] Wieteska E, Ziolek A. Chemia Analityczna, 2000, 45: 325-339.
[3] Lu I Y, Schroeder W H. Water, Air, & Soil Pollution, 1999, 112: 279-295.
[4] Morita M, Edmonds J S. Journal of Pure & Applied Chemistry,1998, 70: 1585-1615.
[5] Rose M., Owen L, Baxter M, et al. Journal of Analytical Atomic Spectrometry, 2001, 16:
1101-1106.
[6] Yang G Y, Zhang C M, Hu Q F, et al. Journal of Chromatographic Science, 2003, 41(4):
195-199.
[7] Ellis A T, Kregsamer P, Potts P J, et al. Journal of Analytical Atomic Spectrometry,
1998, 13 (11): 209R-232R.
[8] Abu Zuhri A Z, Voelter W. Fresenius' Journal of Analytical. Chemistry, 1998, 360: 1-9.
[9] El-Sayed A Y. Analytical Letters, 1998, 31: 1905-1916.
[10] Sang M P, Hee-Seon C. Analytical Chimica Acta, 2002, 459: 75-81.
[11] Chatterjee S, Pillai A, Gupta V K. Talanta, 2002, 57: 461-465.
[12] Gao H W. Asian Journal of Chemistry, 2000, 12: 78-84.
[13] Chen W R, Yang W F, Yang P et al. Chinese Journal of Chemical Reagent (Huaxue Shiji),
1990, 12 (4): 215-217,235.
[14] Hu Q F, Yang G Y, Yin G Y, et al. Talanta, 2002, 57 (4): 751-756.
[15] Mathew.L, Reddy M L P, Prasada-Roa T R.R T, et al. Microchimica Acta, 1997,
127:125-128.
[16] Kiwan A M, EI-Shahawi M S, Aldhaeri S M, et al. Talanta, 1997, 45: 203-211.
[17] Feng Y L, Narasaki H, Tian L C, et al. Analytical Sciences, 1999, 15: 915-918.
[18] Talanova G G, Elkarim N S A, Talanov V S, et al. Analytical Chemistry, 1999, 71:
3106-3109.
[19] Yallouz A V, Calixto-De-Campos R, Paciornik S. Fresenius' Journal of Analytical
Chemistry, 2002, 366 (5): 461-465
[20] Garg B S, Sharma R K, Bhojak N, et al. Microchemical Journal, 1999. 61: 94-114.
[21] Pyrzynska K, Trojanowicz M. Critical reviews in Analytical Chemistry, 1999, 29:
313-321.
[22] Yang G Y, Dong X C, Hu Q F et al. Analytical
Letters, 2002, 35: 1735-1745.
[23] Keil O, Joachim D, Dietrich A V. Journal of
Chromatographic Science, 1997, 35: 519-524.
[24] Haddad P R, Doble P, Macka M. Journal of Chromatography, 1999, 856: 145-177.
胡群1,刘志华1,王建1,胡琳2
(1云南烟草科学研究院, 昆明 650223;2云南大学生命科学学院 昆明 650091)
摘要 本文建立了一种高灵敏度、高选择性和快速测定汞的方法。其基本是利用汞与ABTR快速反应成的络合物可被C18 disk固相萃取小柱富集。在pH 3.5的HAc-NaAc缓冲溶液中,乳化剂-OP存在下,对氨基苯亚甲基硫代若丹宁(ABTR)与汞快速反应生成红色的2:1(汞:ABTR)稳定络合物,可被 C18固相萃取小柱富集并可被二甲基甲酰胺(DMF)稳定洗脱, 其洗脱液富集倍数可达50倍。在DMF介质中, 该络合物的lmax=545 nm,e=1.21×10 5 L.mol-1.cm-1。测定液中汞量在0.01~3.0μg/mL内符合比尔定律。0.1m g/ml 的样品11次重复测定的相对标准偏差为1.69%。本法用于烟草及其添加剂中微量汞的检测可获满意结果。
关键词 汞;固相萃取;分光光度法;对氨基苯亚甲基硫代若丹宁