http://www.chemistrymag.org/cji/2007/098035pe.htm |
Aug.10,
2007 Vol.9 No.8 P.35 Copyright |
Simultaneous Determination of 2-keto-L-Gulonic Acid and 2-keto-D-Gluconic Acid in Fermentation Broth by HPLC
Sun Sufang, Liu Guorui, Wang Yanhuan, Zhang Yan, Liu Cuifen, Song Yongmei
(College of Chemistry and Environmental Science, Hebei University, Baoding 071002, China)
Abstract A simple and fast HPLC method for simultaneous determination of 2-keto-L-gulonic acid and 2-keto-D-gluconic acid in fermentation broth was described. Separation and quantitation were achieved on a shim-pack CLC-NH2 column (150 mm×6 mm, 5 mm) using 0.015 mol/L ammonium dihydrogen phosphate solution (pH 4.1, adjusted by phosphoric acid) as mobile phase. Detection was performed by UV absorbance at a wavelength of 210 nm, and the flow rate was 1 mL/min. The elution of the analytes was achieved in less than 19.0 min. The linearity, accuracy and precision of the method were found to be good and acceptable over the concentration ranges from 10 to 600
mg·mL-1 for both 2-keto-L-Gulonic acid and 2-keto-D-Gluconic acid.Keywords HPLC, 2-keto-L-gulonic acid (2-KLG), 2-keto-D-gluconic acid (2-KDG) 1. INTRODUCTION
Vitamin C is a kind of essential vitamin and antioxidant in human body and it can be widely used in the medical and food industry. In the commercial production of Vitamin, only 2-keto-L-gulonic acid (2-KLG) is a key intermediate. The most popular process for synthesizing 2-KLG is the 70-year-old Reichstein's method, in which glucose is transformed into 2-KLG through five chemical steps[1]. Since Gray's description of microbial methods for the conversion of L-sorbose to 2-KLG, the use of microbial methods has become attractive and gradually replaced the chemical method for lower industrial cost and less ecological problems[2,3]. "2, 5-diketo-D- gluconic acid (2, 5-DKG) pathway" is one of the most commercially promising methods for microbial production of 2-KLG[4], during the course of preparing 2-KLG, 2-keto-D-gluconic acid (2-KDG), the stereoisomer of 2-KLG might be accumulated simultaneously with 2-KLG from other organic acids in the fermentation broth (see Figure 1). Thus, simultaneous identification and quantitative determination of 2-KLG and 2-KDG in fermentation broth was very important in industrial production and scientific research of vitamin C.
In the past years, the identification of 2-KLG was usually performed by paper chromatography method[5], but this method could not be used for the quantitative determination of 2-KLG. The quantitative determination of 2-KLG was usually performed by iodometry method, but the method could not be used for the identification of 2-KLG. In addition, both above methods were complicated to be operated.
A recent literature survey revealed that literatures about the simultaneous determination of 2-KLG and 2-KDG were very less to our knowledge. Eero[6] had applied HPLC with a column packed with sulfonated polystyrene copolymer resin to the analysis of 2-KLG and its related organic acids, this method had a lower theoretical plates yielding relatively overlapping peaks, long run time and poor resolution, which was not good enough for quantitative analysis. Qiao et al[7] demonstrated a HPLC method to determine 2-KLG, in which the refractive index detector was employed and the results obtained presented poor sensitivity, resolution and low recovery. The best method reported was that presented by Choi et al[8], which described a capillary zone electrophoresis method to determine 2-KLG and its related organic acid simultaneously. In this paper, a simple and fast HPLC method was described, which could be used for simultaneous determination of 2-KLG and 2-KDG that were formed in the fermentation process for synthesis of ascorbic acid using an ordinary NH2 column and a simple mobile phase at detection wavelength 210 nm.
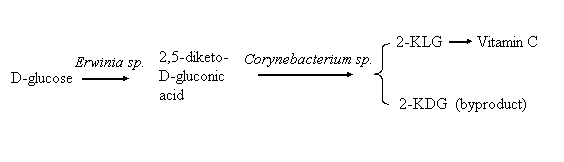
Figure 1 Simple sketch about the transformation of glucose to vitamin C 2. EXPERIMENTAL SECTION
2.1 Apparatus and Reagents
Apparatus: The chromatographic system consisted of a Shimadzu LC-6A liquid delivery pump (Japan), Shimadzu SPD-6AV variable UV/VIS detector (Japan), Shimadzu CR-3A data processor (Japan) and Rheodyne 7125 model injector (USA). PERKIN-ELMER AD-6 autobalance (USA) was used for weighing substances.
Reagents: 2-KLG, 2-KDG standards were purchased from Sigma (St. Louis, MO, USA), Ammonium dihydrogen phosphate, phosphoric acid and other reagents were all of analytical grades. The fermentation broth was kindly provided by Agricultural University of Hebei. Thrice-distilled water was used to prepare all the standard and sample solutions.
2.2 Chromatographic Conditions
Final chromatographic conditions were an isocratic elution with 0.015 mol/L NH4H2PO4 solution (pH 4.1, adjusted by phosphoric acid) on a Shim-pack CLC-NH2 chromatographic column (150 mm×6 mm, 5 mm). The flow rate was 1.0 mL·min-1. The detective wavelength was selected at 210 nm. The mobile phase was filtered through a 0.45 mm membrane filter and degassed before use. Separation was at ambient temperature. Under these chromatographic conditions described above, the retention times of 2-KLG and 2-KDG were 14.3 min and 16.2 min respectively. The chromatogram of standards for them was shown in Figure 2.
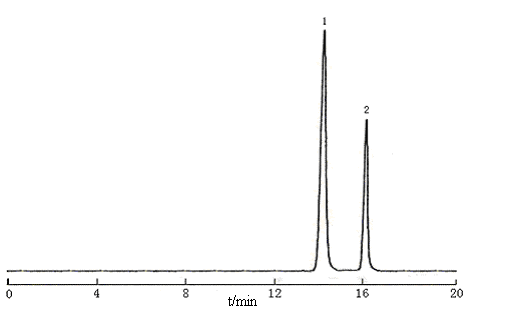
Figure 2 Chromatogram of standards of 2-KLG and 2-KDG
1, 2-KLG; 2, 2-KDG
2.3 Preparation of Standard Solutions
The standard stock solution (1 mg/mL) was prepared by dissolving 25.0 mg 2-KLG and
25.0 mg 2-KDG in a 25 mL volumetric flask and make up to volume with mobile phase. A
series of standard solutions were prepared by quantitatively transferring suitable stock
solution to 10 mL volumetric flasks and made up to the volume with mobile phase.
2.4 Preparation of Sample Solutions
The fermentation broth provided was taken and mixed with ZnSO4 (72.0 mg/mL) and K4Fe(CN)6·3H2O (36.0 mg/mL) according to the proportion of 1:1:1 (v/v/v), and centrifugal precipitation for 10 min by 13000 rpm. Then the above clear solution was filtered through a 0.45 mm Millipore membrane and diluted to three times volume with mobile phase, and then the sample solution was obtained and stored in a refrigerator to be used in the experiments. 3. RESULTS AND DISCUSSION
3.1 The Treatment of the Fermentation Broth
As we all know, the composition of the fermentation broth was very complex, which not only strongly influenced the separation of tested components, but also would damage the chromatographic column to some degree. According to the experiences and some related references, [5,7] the flocculants (ZnSO4 and K4Fe(CN)6·3H2O) was added into the fermentation broth to eliminate the interference of the impurity, varying concentration and proportion of the flocculants was investigated in our experiments, and the results obtained showed that the volume rate of the fermentation, ZnSO4 (72.0 mg/mL) and K4Fe(CN)6·3H2O (36.0 mg/mL) was 1:1:1 as a result of optimization.
3.2 The Selection of Column and the Mobile Phase
We have been engaging in the separation work by HPLC, a lot of experiences were collected in this way. Considering the solubility and the properties of 2-KLG, 2-KDG, we began with CLC-NH2 column and a mobile phase of ammonium dihydrogen phosphate solution as mobile phase with phosphoric acid adjusted to keep the mobile phase in acidic condition. Varying concentrations of NH4H2PO4 solution (0.05-0.4 mol/L) were studied in our experiments, and the results showed that the retention times of 2-KLG, 2-KDG were gradually shortened with the concentration of NH4H2PO4 solution increased and the baseline resolution between them could also be obtained before the concentration of NH4H2PO4 solution increased to 0.015 mol/L, but when the concentration of NH4H2PO4 solution was higher than 0.015 mol/L, the resolution between 2-KLG and 2-KDG was not very good. Besides above, the effect of pH (from 2.9 to 4.9 in increments of 0.4, adjusted with phosphoric acid) on the separation was also investigated, and the results showed that the retention time of both organic acids was obviously shortened with the pH value decreased, so lower pH value was in favor of fast elution of the tested materials, therefore, the total analytical time reduced. However, the baseline resolution between 2-KLG and 2-KDG could not be obtained if the pH value was lower than 4.1. Therefore, taking all things into consideration, 0.015 mol/L NH4H2PO4 solution with phosphoric acid adjusted to keep pH 4.1 was selected finally as the optimum mobile phase for the separation of the organic acids.
3.3 The Selection of Detection Wavelength
The refractive index detector had ever been used to determine 2-KLG, but poor sensitivity was obtained. [7] In this paper, UV/VIS detector was used to determine them. Initial studies were carried out at several wavelengths (190 nm, 193 nm, 205 nm, 210 nm, 215 nm, 220 nm.). It was found that both 2-KLG and 2-KDG had strong absorbance at 210 nm, so a detection wavelength of 210 nm was selected finally.
3.4 System Suitability Test
System performance parameters of the suggested method were determined by analyzing standard working solutions. Chromatographic parameters such as number of theoretical plates (N), resolution (Rs), and capacity factor (k) were determined. The number of theoretical plates were 5438 for 2-KLG and 3890 for 2-KDG, the resolution between 2-KLG and 2-KDG was 3.28, and the capacity factor determined from the retention time relative to the dead time (tM = 2.2 min) were 5.50 for 2-KLG and 6.36 for 2-KDG, respectively. Also, the resolutions between the peak of analyte and the adjacent peak of impurity were all >2.0. From the above data, it could be seen that the described method showed adequate column efficiency, well-separated peaks and good resolution from other peaks and the void volume. Besides above, the repeatability of the system was also determined by six replicate injections of the standard solutions and the RSD calculated from the peak areas were 1.69% for 2-KLG and 1.87% for 2-KDG (n = 6), indicating that the described method showed good repeatability.
3.5 Linearity
The linearity studies were generally performed by preparing standard solutions of six different concentration levels. A proper amount of the standard stock solution was accurately transferred to a 10 mL volumetric flask and diluted with mobile phase to volume, then varying concentration of 2-KLG and 2-KDG(10 mg/mL,50 mg/mL, 100 mg/mL, 200 mg/mL, 400 mg/mL and 600 mg/mL) was obtained. 10 mL of those above solutions were injected in sequence from lower concentration solution to higher concentration solution and the corresponding peak area of both analysts was recorded, each concentration was analyzed three times. Then the regressive curves with the peak area of the tested components as ordinate (Y) and the corresponding concentration (mg/mL) as abscissa (x) were achieved. The results were listed in Table 1. From Table 1, it could be seen that those parameters showed a good linearity with correlation coefficients > 0.999 for each component.
Table 1 Linear regressive equations, linear ranges and correlation coefficients (r) of the two components
Components |
Linear regressive equations |
Linear ranges ( mg/mL) |
r |
2-KLG |
Y=2301.2x-355.5 |
10.0 - 600.0 |
0.9999 |
2-KDG |
Y=2202.1x-1321.5 |
10.0 - 600.0 |
0.9998 |
3.6 Precision
The precision study mainly included two
parts: intra-assay precision and inter-assay precision. The former was determined by
repeatedly analyzing ten aliquots of the standard solution (10
3.7 Limits of Detection and Quantitation
Limit of detection (LOD) was the lowest concentration of analyte in a sample that could be detected under the stated experimental conditions: typically three times of the noise level. The LOD of each active gradient was experimentally determined by six injection of it at its LOD concentration, and the results were found to be 2.7 mg/mL for 2-KLG, 3.1 mg/mL for 2-KDG Meanwhile, Limits of Quantitation (LOQ), which was the lowest concentration of the tested components in a sample that could be determined with acceptable precision and accuracy, was established at a signal-to-noise ratio of 10 and experimentally verified by six injections of each organic acid at its LOQ concentration, the results obtained was 7.8 mg/mL for 2-KLG and 9.1 mg/mL for 2-KDG respectively.
3.8 Solution stability
The stability of both standard and sample solutions were determined by monitoring the peak area of the standard solution of 2-KLG and 2-KDG and sample solution over a period of three days. The results indicated that peak area of 2-KLG and 2-KDG of these solutions remained almost unchanged and no degradation was observed within the given period if these solutions were stored in a refrigerator at about 4 ℃, indicating that these solutions were stable for at least three days.
3.9 Determination of Sample Solution
The sample solution was obtained according to "the preparation of the sample solution" in experimental SECTION, 10 mL of above solution was injected and the peak area was recorded, the results were shown in Figure 3. The concentration of 2-KLG and 2-KDG were calculated to be 82.0 mg/mL and 417.3 mg/mL respectively according to the linearity regression equations.
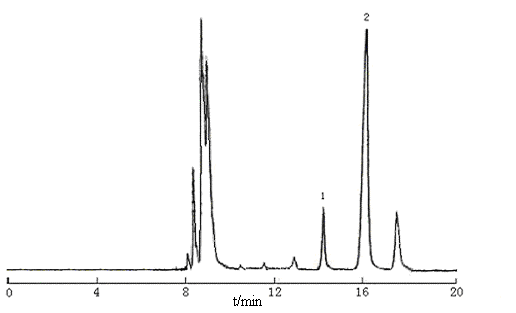
Figure 3 Chromatogram of sample solution
1, 2-KLG; 2, 2-KDG
3.10 Accuracy
The accuracy of a method was the closeness of the measured value to the true value for the
sample. In our experiments, an amount of standards of 2-KLG and 2-KDG were accurately
weighed and added into above known concentration sample solution, and then shaken up. 10
The added mass of 2-KLG and 2-KDG ( mg/mL) |
2-KLG |
2-KDG |
|||
2-KLG |
2-KDG |
Recovery (%) |
RSD(%) |
Recovery (%) |
RSD (%) |
32.8 |
65.6 |
95.7 |
2.73 |
96.5 |
3.04 |
49.2 |
82.0 |
96.4 |
2.49 |
95.2 |
2.68 |
65.6 |
98.4 |
96.8 |
3.01 |
95.4 |
2.87 |
An isocratic HPLC method has been developed for the determination of 2-KLG and 2-KDG in fermentation broth in less than 19.0 min. All the parameters obtained from the experiments for validation show that the proposed method here is reliable and accurate, therefore it not only can be used in the analysis of 2-KLG and 2-KDG in the fermentation process synthesizing ascorbic acid, but also can be extended to the quality control and the monitoring of fermentation broth in biological and biosynthetic laboratories. REFERENCES
[1] Reichstein T, Gruessner A, Oppenauer R. Helvetica Chimica Acta, 1933, 16, 1019-1028.
[2] Boundrant J. Enzyme and Microbial Technology. 1990, 12 (5), 322-329.
[3] Makover S, Ramsey G B, Vane F M, et al. Biotechnology and Bioengineering. 1975, 17 (10), 1485-1514.
[4] Sonoyama T, Tani H, Matsuda K, et al. Applied and Environmental Microbiology. 1982, 43 (5), 1064-1069.
[5] Qiao Ch H, Chen C Sh, Chen F, et al. Industrial Microbiology. 2000, 30 (1), 9-14.
[6] Eero R. [J] Journal of Chromatography, 1982, 218, 695-701.
[7] Qiao Ch H, Yin G L. Modern Scientific Instruments. 2000, 71 (4), 18-19.
[8] Choi O K, Kim C G, Kim Y D, et al. Journal of Chromatography A. 1996, 745, 249-254.
同时测定发酵液中2-酮基-L-古龙酸和2-酮基-D-葡萄糖酸的HPLC的研究
孙素芳,刘国瑞,王燕桓*, 张燕,刘翠芬,宋咏梅
(河北大学化学与环境科学学院 保定071002)
摘要 本文建立了一种简单、快速测定发酵液中2-酮基-L-古龙酸和2-酮基-D-葡萄糖酸的HPLC方法,采用的色谱条件为:shim-pack CLC-NH2色谱柱(150 mm×6 mm, 5 mm),流动相为:0.015 mol/L 磷酸二氢氨 (磷酸调pH为4.1),检测波长为210
nm,流速为:1.0 mL/min。结果表明:2-酮基-L-古龙酸和2-酮基-D-葡萄糖酸在10
- 600 mg·mL-1的浓度范围内,线性关系、精密度和准确度j均较好,单次洗脱时间小于19.0 min,结果令人满意。
关键词 HPLC, 2-酮基-L-古龙酸,2-酮基-D-葡萄糖酸