http://www.chemistrymag.org/cji/2007/098038ne.htm |
Aug.28,
2007 Vol.9 No.8 P.38 Copyright |
Photopromoted carbonylation of alkyl bromides catalyzed by copper powder under ambient conditions
Zou Shunying, Jia Yingping, Yin Jingmei, Gao Dabin, Zhou Guangyun(Liaoning Key Laboratory of Bio-Organic Chemistry, Dalian University, Dalian 116622) Abstract Photopromoted carbonylation of alkyl bromides with carbon monoxide catalyzed by copper powder can be carried out under ambient conditions and the corresponding ester was produced. The yield and the selectivity of ester can be improved by addition of CH3COONa. And after the reaction, the catalyst can be separated easily and reused.
Keywords photopromoted carbonylation, copper powder, alkyl bromides, carbon monoxide, recycle used.
The synthesis of esters by carbonylation is
one of the most important reactions, which usually requires high
temperatures (150-200ºC) and high pressure (10-20 MPa) or precious metal catalysts (Ru,
Rh, Ir)[1]. There are many advantages, however, for photopromoted
carbonylations such as ambient conditions and non-precious metal catalyst [2, 3].
In the previous paper[4], we have reported that the
photopromoted carbonylation of alkyl bromides with CO could be carried out under the
catalysis of copper salts. Notice that the copper salts are soluble in methanol, these
homogeneous catalysts are difficult to be separated after reaction and reused then. Thus,
the use of heterogeneous catalysts is worthy to be considered in the photopromoted
carbonylations.
We report herein that copper powder has been used as catalyst for the
photopromoted carbonylation of alkyl bromides with CO under ambient conditions and the
effect of CH3COONa as additive on the reaction. The catalyst could be recycle
used for several times. To our best knowledge, there are no reports about copper powder as
heterogeneous catalyst in the photopromoted carbonylations of alkyl bromides.
1 EXPERIMENTAL
Carbon monoxide (99.9%, Dalian Guangming Institute); methanol (G.R., Beijing Chemical
Engineering Co.); bromocyclohexane, 1-bromopentane, 1-bromohexane, 1-bromoheptane,
1-bromooctane, 1-bromodedecane, n-decane, copper powder, CH3COONa (A.P.,
Beijing Phentex Chemicals Co., Ltd.).
A typical reaction
procedure was as follows: First, a quartz reactor was equipped with a 400 W high pressure mercury lamp and cooled with circulating cold water
to maintain room temperature. Then a quartz test tube, which contained the methanol
solution of alkyl bromides (100 mmol/L), copper powder (0.1 mmol) and additive, was placed
closely against the outer wall of the quartz reactor. The reactions were carried out with
irradiation for 6 hours at room temperature under 0.1 MPa of CO pressure. The reaction
products were characterized by GC retention time and GC-MS. During the procedure, n-decane
was used as internal standard.
2 RESULTS AND DISCUSSION
The photopromoted carbonylation of
bromocyclohexane with carbon monoxide and methanol catalyzed by
copper powder could be carried out under ambient conditions and cyclo-C6H11COOCH3 was produced (Scheme 1). The results indicated
that the yield of 22% and the selectivity of 31% for cyclo-C6H11COOCH3
could be obtained with copper powder as catalyst and the major byproduct was
cyclohexene.
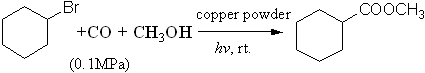
Scheme 1
Furthermore, the effect of different additives on carbonylation of bromocyclohexane with CO was investigated. The results are shown in Table 1.
Table 1 Effect of different additives on photopromoted carbonylation of bromocyclohexane
Additivesa |
Conversion (%) |
Yield (%) |
Selectivity (%) |
None |
71 |
22 |
31 |
HCOONa |
73 |
30 |
41 |
CH3COONa |
66 |
32 |
48 |
NaHSO3 |
60 |
4 |
7 |
p-toluenesulfonic acid |
72 |
4 |
6 |
In order to improve the reactivity, the effect of CH3COONa concentration on the reaction was investigated. The results are shown in Figure 1.
From Figure 1, it can be seen that the yield and selectivity of the ester depended on the amount of CH3COONa when the concentration of CH3COONa was lower than 40mmol/L. For the concentration of CH3COONa was over 40mmol/L, the change of the yield was not obvious, but the selectivity was decreased with the increasing of the byproduct. The best concentration of CH3COONa was 40mmol/L, the yield and the selectivity was 36% and 53%, respectively.
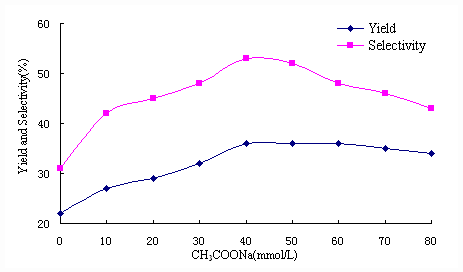
Figure 1 Effect of CH3COONa concentration on photopromoted carbonylation of bromocyclohexane
In addition, the
recycle use of copper powder as catalyst for the carbonylation of bromocyclohexane was
studied. The results are shown in Figure 2.
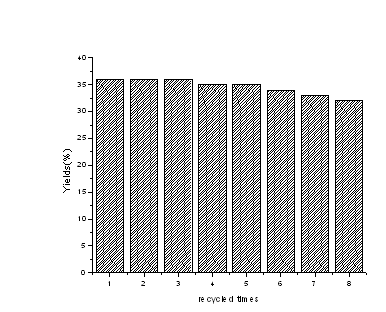
Figure 2 Effect of recycle times of copper powder on
the photopromoted carbonylation of bromocyclohexane with CO
It can be seen that the yield of cyclo-C6H11COOCH3
decreased monotonically from 36% to 32% in the eight recycles. The observations
demonstrate that the catalytic activity of copper powder is not change obviously in the
cycle and the catalysts can be used repeatedly.
Under the same
conditions, the photopromoted carbonylation of linear alkyl bromides has also been
investigated with copper powder (Scheme 2). The results are shown in Table 2.
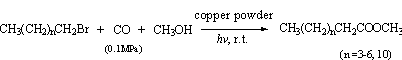
Scheme 2
Table 2 Effect of copper powder on photopromoted carbonylation of different substrates
Conversion (%) |
Yield (%) |
Selectivity (%) |
|
1-bromopentane |
96 |
15 |
16 |
1-bromohexane |
92 |
20 |
22 |
1-bromoheptane |
96 |
26 |
27 |
1-bromooctane |
90 |
20 |
22 |
1-bromodedecane |
92 |
16 |
17 |
It can be seen that copper powder can also catalyze photopromoted carbonylation of linear alkyl bromides with CH3COONa as additive, and the corresponding ester was obtained. Further research on mechanism is underway.
Acknowledgment The authors are indebted to the National Natural Science Foundation of China (No.20372012) for the generous financial support.
REFERENCES
[1] Tao Y T. Chow T J. Lin J T et al.. J. Chem. Soc. Perkin Trans. I, 1989, 1:
2509-2511.
[2] Tao Y T. Yeh W L. Chow Y L et al.. J. Mol. Catal., 1991, 67: 105-115.
[3] Sun Y. Yin J M. Gao D B et al.. Chin .Chem. Lett., 2003, 14(6): 575-578.
[4] Wen T. Jia. Y P. Gao D B et al.. Chin .Chem. Lett., 2005,16(11): 1455-1458
[5] Cash D. Combs A. Dragojlovic V. Tetrahedron Lett., 2004, 45: 1143-1145.
光促进温和条件下铜粉催化溴代烷烃的羰基化反应
(大连大学生物有机省重点实验室,大连 116622)
摘要 在温和条件下,以铜粉为催化剂,实现了溴代烷烃与一氧化碳的光促进羰基化反应,得到相应的羧酸甲酯。添加物醋酸钠对反应有促进作用,反应后,催化剂铜粉易于分离,可重复使用。
关键词 光促进羰基化,铜粉,溴代烷烃,一氧化碳,重复使用