http://www.chemistrymag.org/cji/2007/099043pe.htm |
Sep.30,
2007 Vol.9 No.9 P.43 Copyright |
The reaction of aromatic aldehydes and 1,3-cyclohexanedione catalyzed by PEG-400 in water
Wang Aiqinga , Cheng Zhaolib,
Wang Jinga, Li Jiangqiaoa, Jin Tongshoua
(aCollege of Chemistry and Environmental Science, Hebei University, Baoding
071002; b Bureau of Hydrology & Water Resources Survey,Hebei Baoding 071000,China)
Abstract Synthesis of
9-aryl-1,8-dioxooctahydroxanthene derivatives and 2,2’-arylmethylene bis(3-hydroxy- 2-cyclohexene-1-one) from aromatic
aldehyde and 1,3–cyclohexadione catalyzed by
polyethylene glycol 400 (PEG-400) in water was described. This method provides several
advantages such as environment friendliness, high yields and simple work-up procedure.
Moreover, water was chosen as a green solvent.
Keywords Aromatic aldehyde 1, 3-cyclohexadione Water PEG-400
In the past decade, there has been growing recognition that water is an attractive medium for many organic reactions not only for the advantage accorded by avoiding extensive drying reactants, catalyst and solvent, but also for the unique reactivity and selectivity that some times result.1, 2 On the other hand, organic reaction in water without using harmful organic solvents are the current great interest especially in relation to today environmental concerns. However, water as a solvent was not frequently used until recently for several reasons such as many organic materials do not dissolve in water and many reactive intermediates and catalysts are decomposed in water. So it is necessary for adding some phase-transfer catalysis (PTC) or surfactant such as hexadecyltrimethylammonium bromide (HTMAB), PEG-400, tetrabutylammonium bromide (TBAB), 4-dodecylbenesulfonic acid (DBSA), because they benefit the organic materials uniform dispersion in water in the course of synthesis. Based on our recent research, we have developed novel routes for the synthesis of some hetreocyclics compounds catalyzed by these PTC or surfactant in water.3 Our important aim of this research is to synthesize more hetreocyclics compounds in media aqueous as well.
It has been reported that the reaction of aromatic aldehyde and 1,3-cyclohexanedione can yield 9-aryl-1,8-dioxooctahydroxanthene and their derivatives and 2,2’-arylmethylene bis(3-hydroxy-2-cyclohexene-1-one) by many methods.4-7 However, the use of PEG-400 as the catalyst in aqueous media for the synthesis of them has not been reported. In this manuscript, the authors wish to report a general and highly efficient route for the synthesis of these two produces and their derivatives using PEG-400 as catalysts. This is an efficient synthesis method, not only preserves the simplicity but also consistently gives the corresponding products in good yields (Scheme 1).
2 RESULTS AND DISCUSSION
When the aromatic aldehyde 1, 1, 3-cyclohexadion 2
was performed in water in the presence of PEG-400 and NH2SO3H, high
yields of products 3 and 4 were found. The results are summarized in Table 1
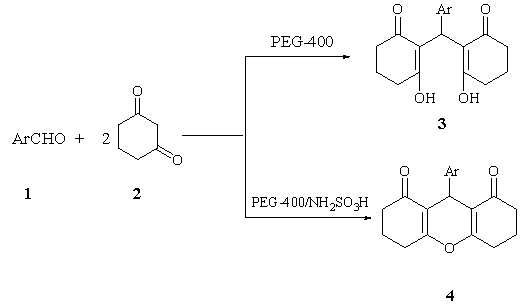
Scheme 1
Table 1 Synthesis of 9-aryl-1,8-dioxooctahydroxanthene derivatives and 2,2'-aryl-methylene bis(3-hydroxy-2-cyclohexene-1-one) in aqueous media by using PEG-400 or PEG-400 /NH2SO3H
Entry |
Ar |
Product |
Yielda(%) |
M. P./ oC |
|
Found |
Reported |
||||
1 |
C6H5 1a |
3a |
81 |
206-208 |
205-207 |
4a |
84 |
268-270 |
270-271 |
||
2 |
4-ClC6H41b |
3b |
85 |
202-203 |
202-204 |
4b |
82 |
285-287 |
289-291 |
||
3 |
3-ClC6H41c |
3c |
81 |
187-191 |
|
4c |
83 |
270-272 |
276-277 |
||
4 |
3,4-Cl2-C6H31d |
3d |
87 |
206-209 |
|
4d |
77 |
204-208 |
229 |
||
5 |
4-NO2C6H4 1e |
3e |
82 |
194-197 |
191-192 |
4e |
86 |
260-261 |
263 |
||
6 |
3-NO2C6H4 1f |
3f |
82 |
196-198 |
|
4f |
86 |
282-283 |
286-288 |
||
7 |
2-NO2C6H4 1g |
3g |
83 |
194-196 |
|
4g |
82 |
239-241 |
245-246 |
||
8 |
4-HOC6H4 1h |
3h |
80 |
200-205 |
|
4h |
85 |
275-278 |
279-281 |
||
9 |
4-HO-3-CH3OC6H31i |
3i |
80 |
188-191 |
|
4i |
79 |
243 |
245-246 |
||
10 |
4-CH3C6H4 1j |
3j |
84 |
187-189 |
|
4j |
77 |
256-257 |
262-263 |
||
11 |
4-CH3OC6H4 1k |
3k |
83 |
185-188 |
178-180 |
4k |
79 |
198-199 |
201-202 |
||
a
Isolated yieldAs shown in Table 1, the different
products were obtained using different catalyst in this reaction. In a typical general
experimental procedure, aromatic aldehyde 1 and 1,3-cyclohexanedione 2 were
grinded in the presence of PEG-400 or PEG-400/NH2SO3H, the
corresponding products 3 and 4 were obtained in good to excellent yields.
The catalyst effect shows that acid is needed during the cyclization. No strongly
obvious effect of electron and nature of substituents on the aromatic ring were observed.
All aromatic aldehydes containing electron-withdrawing groups (such as nitro group,
halide) or electron-donating groups (such as hydroxyl group, alkoxyl group) were employed
and reacted well to give the corresponding products 3 and 4 in good to
excellent yields under this reaction conditions.
Taking the reaction of 3-chlorobenzaldehyde as an example, we
investigate the effect of the catalyst reagents on the reaction. It was found that the
catalyst plays a crucial role in the success of the reaction in terms of the rate and the
yields. For example, the reaction could be carried out in the absence of PEG-400 when the
mixture (1c and 2) in water, but it obtained very poor yield (46%).
Increasing of the catalyst to 200mg,300mg,350mg,400mg, it results in
accelerating the reaction with the yield to 80%、91%、90%、80.3% respectively. Use
of just in refluxing water is sufficient to push the reaction forward. Higher amounts of
the catalyst did not improve the results to a greater extent. Thus, 300mg PEG-400 was
chosen as a quantitative catalyst for these reactions. At the results, the best use level
of PEG-400 and NH2SO3H was 300mg/40mg.
3 CONCLUSION
In summary, a novel and efficient procedure for the synthesis of 9-aryl-1,8-dioxo-
1,2,3,4,6,7,8-octahydroxanthenes and 2,2’-arylmethylene
bis(3-hydroxy-2-cyclohexene-1-one) through the reaction of aromatic aldehydes and 1,
3-cyclohexanedione using PEG-400 or PEG-400/ NH2SO3H as catalyst has
been reported. This is a one–pot condensation in
water. It is noteworthy that water solution is a clean and environmentally desirable
system. No harmful organic solvents are used. This report has proposed and demonstrated a
new useful and attractive process for the synthesis of these compounds.
4 EXPERIMENTAL
Liquid aldehydes
were distilled before use. IR spectra were recorded on a Bio-Rad FTS-40 spectrometer
(KBr). 1H NMR spectra were measured on a Bruker AVANCE 400 (400 MHz) spectrometer using
TMS as internal reference and DMSO as solvent. Melting points are uncorrected.
Procedure for the synthesis of 3 and 4 using PEG-400 or PEG-400/ NH2SO3H
as catalyst
A mixture of an aromatic aldehyde 1 (1.0 mmol), 1, 3-cyclohexanedione 2 (2.0 mmol) and
PEG-400 (300mg) or PEG-400 / NH2SO3H (300mg/40mg) in water (20 mL)
was stirred at refluxing for three hours. The progress of the reaction was monitored by
thin layer chromatograph. After completion of the reactions, the mixture was cooled to
room temperature and solid was filtered off and washed with H2O (40 mL) and the
crude products were got. The crude products 3 and 4 were purified by recrystallization by
95% ethanol. Data of some compounds are shown below:
3a. IR (KBr):nmax
3425, 3060, 2954, 2870, 2633, 1598, 1468, 1419, 1375, 1248, 1149, 1066, 974, 870, 789 cm-1.
dH 1.99 (m, 4H,
2 × CH2), 2.30-2.51 (m, 8H, 4 × CH2), 5.41 (s, 1H, CH), 7.10-7.28
(m, 5H, Ar-H), 12.15 (br, s, 1H, OH), 12.19 (br, s, 1H, OH).
3b. IR (KBr):nmax
3505, 3050, 2945, 2870, 2633, 1589, 1486, 1421, 1357, 1248, 1145, 1061, 870, 798 cm-1.
dH 1.98(m, 4H, 2
× CH2), 2.30-2.48 (m, 8H, 4 × CH2), 5.39 (s, 1H, CH), 7.21-7.28
(m, 4H, Ar-H), 12.23 (br, s, 2H, OH).
3c. IR (KBr):nmax
3424, 3058, 2954, 2868, 2634, 1596, 1468, 1418, 1375, 1307, 1248, 1151, 1066, 974, 890,
870, 789 cm-1. dH
2.01 (m, 4H, 2 × CH2), 2.31-2.51 (m, 8H, 4 × CH2), 5.50 (s, 1H,
CH), 6.98-7.28 (m, 4H, Ar-H), 11.57 (br, s, 1H, OH), 11.91 (br, s, 1H, OH).
3d. IR (KBr):nmax
3425, 3060, 2950, 2865, 2630, 1601, 1470, 1417, 1379, 1250, 1152, 1135, 975, 889, 790 cm-1.
dH 2.03(m, 4H, 2
× CH2), 2.30-2.52 (m, 8H, 4 × CH2), 5.51 (s, 1H, CH), 6.98-7.23
(m, 3H, Ar-H), 11.57 (br, s, 1H, OH), 11.93 (br, s, 1H, OH).
3e. IR (KBr):nmax
3424, 3085, 2945, 2886, 2643, 1595, 1462, 1375, 1307, 1248, 1151, 1066, 974, 890, 870, 798
cm-1. dH
2.05 (m, 4H, 2 × CH2), 2.32-2.48 (m, 8H, 4 × CH2), 5.50 (s, 1H,
CH), 6.99-7.38 (m, 4H, Ar-H), 12.07 (br, s, 1H, OH), 12.19 (br, s, 1H, OH).
3f. IR (KBr):nmax
3424, 3087, 2955, 2812, 2372, 1654, 1523, 1418, 1350, 1202,1131, 1074, 1015, 960, 860,
815, cm-1. dH
1.99 (m, 4H, 2 × CH2), 2.35-2.50 (m, 8H, 4 × CH2), 5.50 (s, 1H,
CH), 6.99-7.38 (m, 4H, Ar-H), 12.01 (br, s, 1H, OH), 12.15 (br, s, 1H, OH).
3g. IR (KBr):nmax
3424, 3077, 2964, 2945, 2896, 2873, 1678, 1619, 1520, 1335, 1202, 1178, 1130, 823, 787cm-1.
dH 2.02 (m, 4H,
2 × CH2), 2.36-2.52 (m, 8H, 4 × CH2), 5.42 (s, 1H, CH), 7.02-7.38
(m, 4H, Ar-H), 12.09 (br, s, 1H, OH), 12.18 (br, s, 1H, OH).
3j. IR (KBr): nmax
3434, 3057, 3031, 2953, 2892, 2820, 1658, 1616, 1510, 1360, 1201, 1175, 1126,
819, 785 cm-1; dH
1.93~2.00 (m, 4H, 2 × CH2), 2.25~2.34 (m, 8H, 4×CH2), 3.75 (s, 3H,CH3O), 5.39
(s, 1H,CH) , 6.99-7.38 (m, 4H, Ar-H), 12.07 (br, s, 1H, OH), 12.19 (br, s, 1H, OH).
3k. IR (KBr):nmax
3424, 3061, 2960, 2870, 2628, 1605, 1471, 1414, 1380, 1252, 1170, 1140, 1000, 890, 840,
790, 750, 715 cm-1. dH 1.99 (m, 4H, 2 × CH2), 2.31-2.51 (m, 8H, 4
× CH2), 5.47 (s, 1H, CH), 2.78 (s, 3H, CH3Ar), 6.92-7.21 (m, 3H,
ArH), 11.57 (br, s, 1H, OH), 11.91 (br, s, 1H, OH).
4a. IR (KBr):nmax
2949, 2887, 2371, 1653, 1491, 1451, 1418, 1236, 1203, 1176, 1132, 1006, 958, 907, 830,
779, 702, 614, 538 cm-1. dH 1.81~1.90(m, 2H,
CH2), 1.92~1.98 (m, 2H, CH2),
2.26~2.31 (m, 4H, 2×CH2), 2.61~2.68 (m, 4H, 2×COCH2), 4.59 (s,1H, CH), 7.12~7.22 (m, 5H, ArH);
4b. IR (KBr):nmax
3301, 3089, 3048, 2950, 2923, 2889, 2866, 2361, 1669, 1618, 1487, 1421, 1359, 201,1173,
1128, 1011, 958, 907, 836, 767, 740, 689, 608, 533 cm-1. δH 1.83~1.90 (m,
2H, CH2), 1.93~1.99 (m, 2H, CH2),
2.25~2.34 (m, 4H, 2×CH2), 2.61~2.70 (m, 4H, 2×COCH2), 4.56 (s, 1H,CH) , 7.20 (d, 2H, J=8.4 Hz , ArH), 7.26 (d, 2H, J=8.4 Hz, ArH).
4c: IR (KBr): nmax
2953, 2890, 1672, 1621, 1474, 1358, 1201, 1174, 1129, 847, 684 cm-1; dH 7.13 (4H, m, ArH), 4.56
(1H, s, CH), 2.64 (4H, m, 2×COCH2), 2.29 (4H, m, 2×CH2), 1.95 (2H,
m, CH2), 1.88 (2H, m, CH2).
4d. IR (KBr):nmax
3087, 2955, 2887, 2812, 1654, 1621, 1523, 1418, 1350, 1202, 1173, 1131, 1074, 1015, 960,
908, 860, 815, 717, 669, 624, 535 cm-1. dH.179-1.89 (m, 2H, CH2), 1.91~1.99 (m, 2H, CH2), 2.19~2.33 (m, 4H, 2×CH2), 2.55~2.70 (m, 4H, 2×COCH2), 4.78 (s, 1H, CH), 7.19~7.22 (m, 1H, ArH), 7.25~7.31
(m, 2H, ArH).
4e. IR (KBr):nmax
295, 3113, 3081, 3043, 2948, 2895, 2870, 2818, 2446, 1665, 1607, 1521, 1422, 348,1202,
1174, 1128, 1008, 958, 906, 818, 720, 688, 604, 533 cm-1. δH 1.82~1.92 (m,
2H, CH2), 1.95~2.01 (m, 2H, CH2),
2.22~2.37 (m , 4H, 2×CH2), 2.59~2.74 (m, 4H, 2×COCH2), 4.68 (s, 1H, CH), 7.49 (d, 2H, J=8.8 Hz, ArH), 8.09 (d, 2H, J=8.8Hz, ArH).
4f. IR (KBr):nmax
3087, 2955, 2887, 2812, 2372, 1654, 1621, 1523, 1418, 1350, 1202, 1173,1131, 1074, 1015,
960, 908, 860, 815, 717, 669, 624, 535 cm-1. dH 1.83~1.92 (m,
2H, CH2), 1.95~2.01 (m, 2H, CH2),
2.23~2.37 (m, 4H, 2×CH2), 2.64~2.75 (m, 4H, 2×COCH2), 4.69 (s, 1H, CH), 7.52~7.56 (m, 1H, ArH), 7.65~7.67
(m, 1H, ArH), 7.99~8.03 (m, 2H, ArH).
4g. IR (KBr): nmax
3077, 2964, 2945, 2896, 2873, 1678, 1619, 1520, 1335, 1202, 1178, 1130, 823, 787 cm-1;
1dH
7.77 (1H, d, J=6.8Hz, ArH), 7.56 (1H, m, ArH), 7.35 (2H, d, J=6.8Hz, ArH), 5.40 (1H, s,
CH), 2.64 (4H, m, 2×COCH2), 2.27 (4H, m, 2×CH2), 1.95 (2H, m, CH2),
1.86 (2H, m, CH2).
4h. IR (KBr): nmax
3379, 3021, 2949, 2921, 2892, 1662, 1612, 1515, 1361, 1207, 1173, 1131, 835, 762 cm-1;
dH 9.19 (1H, s,
OH), 6.95 (2H, d, J=8.4Hz, ArH), 6.59 (2H, d, J=8.4Hz, ArH), 4.48 (1H, s, CH), 2.63 (4H,
m, 2×COCH2), 2.27 (4H, m, 2×CH2), 1.94 (2H, m, CH2),
1.86 (2H, m, CH2).
4i. IR (KBr):nmax3340,
2949, 2832, 2344, 1666, 1645, 1512, 1359, 1274, 1173, 1125, 1038, 951, 907, 852, 808, 735,
625, 538 cm-1. dH
1.80~1.91 (m, 2H, CH2), 1.92~2.10 (m, 2H, CH2), 2.28~2.31 (m, 4H, 2×CH2), 2.5~2.71 (m, 4H, 2×COCH2), 3.71 (s, 3H, OCH3),
4.51 (s, 1H, CH), 6.50~6.53 (m, 1H, ArH),6.59~6.61 (m, 1H, ArH), 6.74~6.75
(m, 1H, ArH), 8.71(s, 1H, OH).
4j. IR (KBr): nmax
3057, 3031, 2953, 2892, 2820, 1658, 1616, 1510, 1360, 1201, 1175, 1126, 819, 785 cm-1;dH 7.00 (4H, dd, J1=22Hz,
J2=8Hz, ArH), 4.54 (1H, s, CH), 2.60 (4H, m, 2×COCH2), 2.25 (4H, m,
2×CH2), 1.93 (2H, m, CH2), 1.84 (2H, m, CH2).
4k. IR (KBr):nmax
3027, 2956, 2896, 2830, 2368, 1663, 1660, 1510, 1466, 1361, 1239,1176, 1131, 1033, 955,
904, 839, 758, 672, 612, 534 cm-1. dH 1.80~1.90 (m,
2H, CH2), 1.95~1.98 (m, 2H, CH2),
2.27~2.30 (m, 4H, 2×CH2), 2.61~2.65 (m, 4H, 2×COCH2), 3.68 (s, 3H, OCH3),
4.53 (s, 1H, CH), 6.77 (d, 2H, J=8.4 Hz,
ArH), 7.08 (d, 2H, J=8.4 Hz, ArH).
REFERENCES
[1] C. J.Li, T. H. Chan, Organic Reaction in Aqueous Media, John Wiley& Sons Inc: New
York, 1997.
[2] (a) R. Breslow, U.Maitra, D.Rideout, Tetrahedron Lett. ,1983, 24, 1901. (b) S. D.
Copley, J. R.Knowles, J. Am. Chem. Soc., 1987, 109, 5008.
[3] (a) T. S.Jin, J. C.Xiao, S. J.Wang, T. S.Li, X. R.Song, Synlett ,2003, 2001. (b) T. S.
Jin, A. Q.Wang, X.Wang, J. S.Zhang, T. S. Li, Synlett ,2004, 871. (c) T. S.Jin, J.
S.Zhang, J. C. Xiao, A. Q.Wang, T. S.Li, Synlett, 2004, 866. (d) Jin, T. S.; Wang, A.Q.;
Cheng, Z. L.; Zhang, J. S.; Li, T. S. Synth. Commun. 2005, 35, 2339. (e) Jin, T. S.; Liu,
L. B.; Zhao, Y.; Li, T. S. Synth. Commun. 2005, 35, 2379. (f) Jin, T. S.; Wang, A. Q.;
Cheng, Z. L.; Zhang, J. S.; Li, T. S. Synth. Commun. 2005, 35,137. (g) Jin, T. S.; Liu, L.
B.; Zhao, Y.; Li, T. S. Synth. Commun. 2005, 35, 1859. (h) Jin, T. S.; Liu, L. B.; Zhao,
Y.; Li, T. S. J. Chem. Research(s) 2005, 162.
[4] Hua G P, Li T J, Zhu S L, Zhang X J Chin. J. Org. Chem., 2005, 25(6), 716.
[5] Jonathan R D, Srinivas K R, Glen E B Eur. J. Med. Chem., 1988, 23, 111.
[6] Shanmugasundaram P, Prabahar K J, Ramakrishnan V T J. Heterocyclic. Chem., 1993, 30,
1003.
[7] Chen X D, Xu F P Journal of Shang Hai University (natural science), 2000, 6, 375.
王爱卿a,程朝立b, 王静a,李江乔a,靳通收a
(a河北大学化学与环境科学学院 保定 071002,b河北保定水文水资源勘测局 保定,071000)
摘要 以芳香醛和1,3-环己二酮为原料,以聚乙二醇-400(PEG-400)为相转移催化剂,在水中合成了一系列四酮类化合物;再以聚乙二醇-400(PEG-400)和氨基磺酸为复合催化剂,在水中合成了氧杂蒽类化合物。本实验采用的方法具有操作简单,催化剂价廉易得,对环境友好,产率较高等优点。
关键词 芳香醛 1,3-环己二酮 水相 聚乙二醇