http://www.chemistrymag.org/cji/2007/09a044pe.htm |
Oct.14,
2007 Vol.9 No.10 P.44 Copyright |
Dai Honghai, Yang Liting, Lin Bo, Yi Aihua, Shi Guang
(School of Chemistry and Environment, South China Normal University GuangZhou
510631, China)
Keywords Epoxidation; Kinetics; Soybean oil; Peroxyacetic acid 1 INTRRODUCTION
Epoxides of all kinds of plant oils are well known commercially since the they can undergo many important reactions. Epoxidation of long chain olefins, and unsaturated fatty acid derivatives such as soybean oil and other plant oils have been carried out on an industrial scale [1]. Nowadays, one of the most important epoxidized vegetable oils is the epoxidized soybean oil (ESO) and its worldwide production is about 200 000 t/year [2]. Fatty epoxides are used directly as plasticizers that are compatible with polyvinyl chloride (PVC) and as stabilizers for PVC resins to improve flexibility, elasticity, and toughness and to impart stability of polymer towards heat and UV radiation. Due to high reactivity of the oxirane ring, epoxides also act as a reactant raw material for a variety of chemicals, such as alcohols, glycols, alkanolamines, carbonyl compounds, olefinic compounds and polymers like polyesters, polyurethanes (PU) and epoxy resins.
The modified soy-based vegetable oil polyols could be incorporated as a replacement for conventional polyols, reacting with Toluene dicocyanate (TDI) and 4,4'-Diphenylmethane dicyanate (MDI), to produce flexible slabstock polyurethane foams, elastomers, coating. Soybean oil (SBO) is highly hydrophobic, so an excellent weather stability of the derived PU can be expected, the thermal and oxidative stability of the soybean oil-based PU are comparable with those of the polypropyleneoxide-based PU [3-6].
As the demands of energy increase and fossil fuel reserves are limited, there has been a growing interest in the utilization of renewable resources as an alternative to petroleum based polymers. Consequently, much attention has been focused on the development of polymeric materials from vegetable oils, a sustainable resource [7–9]. SBO which is readily available and is a comparatively inexpensive material can be used to synthesize various types of polymers.
There are four known technologies to produce epoxides from olefinic type of molecules: [a] epoxidation with percarboxylic acids [10], the most widely used in industry, can be catalyzed by acids or by enzymes [1,11]; [b] epoxidation with organic and inorganic peroxides which includes alkaline and nitrile hydrogen peroxide epoxidation as well as transition metal catalyzed epoxidation [12]; [c] epoxidation with halohydrins, using hypohalous acids (HOX) and their salts as the reagents for the epoxidation of olefins with electron deficient double bonds [10]; and [d] epoxidation with molecular oxygen [10].
But during the ways in the epoxidation of vegetable oils, the available technologies that need to be explored are only [a] and [b] described above, namely, epoxidation with percarboxylic acids and epoxdation with organic and inorganic peroxides [13] they are clean and efficient. These technologies can be rendered cleaner by using heterogeneous catalysts replacing traditional homogeneous ones [14]. In the present work the kinetics of epoxidatin of SBO by PAA generated in situ and the relation between the extent of epoxidation and temperature was investigated.
2 EXPERIMENTAL
2.1 Materials
The refined soybean oil with an iodine value 127.23 (g I/100g oil) was kindly provided
by Nanhai Oil Co. Ltd. Glacial acetic acid (99.5%), sodium carbonate, sodium hydroxide,
hydrogen peroxide (30%) and sulphuric acid (98%) are AR grade and purchased from Guangzhou
Chemical Reagent Co.. They were used as received.
2.2 Epoxidation of soybean oil
Epoxidation of SBO was carried out in a 1000-ml four-necked round-bottom flask equipped
with a thermometer sensor, a mechanical stirrer, a condenser, and an isobaric funnel. The
whole apparatus was kept in a water bath to maintain the reaction temperatures at 45, 65,
75 ± 1
The samples were poured into a separating funnel the aqueous layer was drawn off and the oil layer was washed successively with warm dilute sodium carbonate solution until the pH was neutral, and then washed with saturated sodium chloride solution and distilled water. The solvent was removed by using a rotary evaporator, with the help of a water vacuum pump. The oil phase was further dried on anhydrous magnesium sulfate and then filtered. The hydroxyl values of the polyols were determined according to the ASTM E 1899-97 standard test method for hydroxyl groups using reaction with p-toluenesulfonyl isocyanate (TSI) and potentiometric titration with tetrabutylammonium hydroxide. The Epoxy Oxygen Content (EOC) in polyols was determined by direct titration of epoxy groups with HBr according to the standard method for oils and fats. [15].
2.3 Method and test apparatus
The IR spectra were recorded on a PerkinElmer Specrum-1000 Fourier transform infrared (FTIR) spectrometer. Samples were prepared as thin films on KBr plates. The oxirane ring formation reactions were monitored by the appearance of the oxirane C—O double peaks at 823 cm-1and 833 cm-1. 3 RESULT AND DISCUSSION
3.1 FTIR Spectra of SBO and ESBO
The FTIR spectra of SBO and ESBO are shown in the Figure 1, and 2. As it can be seen from the FTIR spectrum of the SBO (Figure 1 and Figure 2), the characteristic peak at 3009 cm-1 was attributed to the C–H stretching of SBO C=C–H. The peak at 3009 cm-1 decreased after the epoxidation reaction and disappeared almost completely after 6 hours reaction at 65 and 75 oC, indicating that almost all the C=C bands have taken part in the epoxidation reaction. The peaks due to C=O stretching vibrations at 1725 cm-1 remained unaltered after the epoxidation reaction. The presence of new peak in the FTIR spectra of ESBO at 823 cm-1 and 833 cm-1, attributed to epoxy group, corroborated the conclusion that the success of the epoxidation reaction of SBO. The other new peak at 3463 cm-1 was attributed to the hydroxyl O–H stretching, indicating that the epoxy group might be opened. The extent of hydroxyl group of ESBO increases with an increase on reaction temperature at the same reacting time.
Figure 1 FTIR spectra: spectra of SBO and ESBO prepared at various temperature reacting for 6 hours
Figure 2 FTIR spectra: part spectra of SBO and ESBO prepared at various temperature reacting for 6 hours
3.2 Effect of temperature on epoxidation
The extents of epoxidation of SBO by PAA generated in situ at various temperatures are
showed in Figure 3. The results indicated that increasing temperature showed a favorable
effect on the extent of epoxidation of SBO by PAA generated in situ. The initial increase
in the extent of epoxidation of SBO with reaction time reached maximum values (for
epoxidation at 75 oC) , and then decreased with further increase in reaction
time, indicating that the epoxy group was opened more and more seriously with further
reaction time at 75 oC. The decrease in the extent of epoxidation value at 75 oC
was at long reaction time very obvious, while the extent of epoxidation value at 65 oC
hardly decreased during initial 8h. Reaction at lower temperature showed lower rate but
gave more stable oxirane ring (for epoxidation at 45 oC).
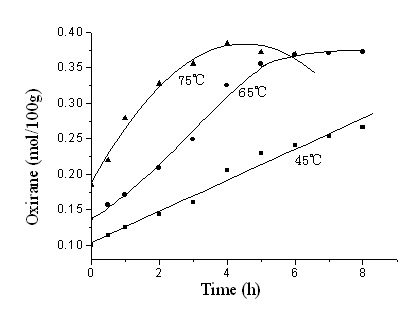
Figure 3 Extent of epoxidation of SBO by peroxyacetic
acid at various temperatures
The epoxy oxygen content, iodine value and OH value of the epoxidation products at 75 oC, 65 oC and 45 oC for 6 hours were shown in Table 1. As can be seen from Table 1, EOC increases with increasing reaction temperature while iodine value decrease. The OH value, due to oxirane cleavage, also increases with increasing of reaction temperature. These results suggested that the maximum optimum level of epoxidation could be obtained in a shorter time at moderate reaction temperature range of 65 and 75oC, at which the rate of epoxide degradation was not high relatively. Consequently, only the initial stage of the reaction used for the kinetic study.
Table 1 Chemical properties of epoxidation products at different temperatures
45 oC |
65 oC |
75 oC |
|
EOC, mol/100 g |
0.240 |
0.350 |
0.378 |
Iodine Value, g I/100 g oil |
60.50 |
26.65 |
12.93 |
OH Value, mg KOH/g |
6.44 |
19.16 |
33.88 |
3.3 Kinetics of epoxidation
The in situ
epoxidation reaction generally takes place in two steps (i) formation of peroxyacid and (ii) reaction of peroxyacid with the
unsaturation [16].
(i) Formation of peroxy acid
![]()
(ii)Epoxidation
If the first step is considered to be the rate determining and the concentration of peroxy acid is assumed to be constant throughout the reaction, then the rate of epoxidation will be given by the following expression [17],
Where, subscript 0 denotes initial concentrations and EP denotes epoxides.
Hence,
㏑{[H2O2]0 - [EP]} = -k·[RCOOH]0·t + ㏑[H2O2]0 (2)
According to Eq.(2), plot of {[H2O2]0 - [EP]} vs. time should yield straight lines for those reactions with negligible degradation of oxirane, and so the rate constants were obtained with the initial slopes. Figure 4 shows the plots for in situ epoxidation of SBO at different temperatures. The rate constants obtained for the reaction were of the order of 10-6mol-1s-1 (Table 2). As the epoxidation reagent (PAA) had been formed, it is mixed with SBO to prepare ESBO; then the rate of the epoxidation of SBO by PAA was greater than the published values [18]. The rate constants in situ epoxidation of SBO by PAA increased with temperature (Figure 5), and the activation energy was determined to be 43.11kJ·mol-1. The value of activation energy was smaller than the published values of 63.26 and 65.69 kJ·mol-1 [17, 18] for the reasons discussed above.
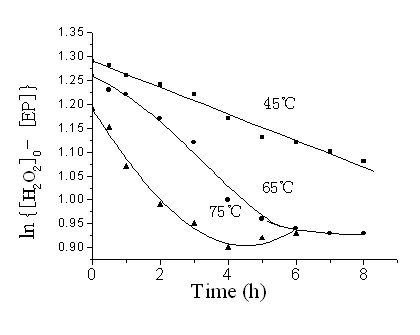
Figure 4 Kinetics of epoxidation of SBO
by PAA
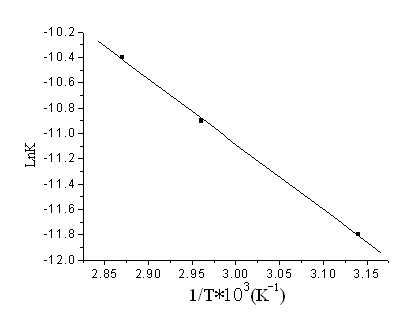
Figure 5 Activation energy, Ea for the
epoxidation of SBO by PAA
Table 2 Rate constants of SBO epoxidation at various temperatures.
Temperature(oC) |
Rate constant of epoxidation K·106(1 mol-1s-1) |
45 |
7.4 |
65 |
18.0 |
75 |
29.6 |
3.4 Thermodynamics of Epoxidation of SBO
The enthalpy of activation,
DH=E-RT (3)
Enthalpy of activation was found to be 45.59 kJ·mol-1.
The average energy of activation, DS, and free energy of activation, DF,
were obtained using the relationship (4) in [20]:
Where, k is the rate constant; R, gas constant; T, absolute temperature; N, Avogadro constant; and h, Plank' constant.
The average values of the thermodynamic parameter were found to be
DS=-208.80 J·mol-1and DF =107.84 kJ·mol-1.
4 CONCLUSIONS
The results from this study show that the epoxidation of SBO by PAA generated in situ
can be carried out at moderate temperatures with minimum epoxide degradation. The kinetic
and thermodynamic parameters of epoxidation obtained indicate that an increase in the
process temperature would promote the rate of epoxide formation and useful for the
scale-up production of ESBO in situ technique.
ACKNOWLEDGMENTS The authors are grateful to the Natural Science Foundation of Guangdong Province (No. 06025028) for financial support.
REFERENCES
[1] Rios, L.A., Weckes, P., Schuster, H., et al. J. Catal., 2005, 232: 19–26
[2] Klaas, M.R., Warwel, S. Ind. Crops Prod., 1999, 9: 125–132
[3] Guo A., Javni I., Petrovic Z.S. J. Appl. Polym. Sci., 2000, 77:467-473
[4] Guo A., Cho Y., Petrovic Z.S. J. Polym. Sci., Part A: Polym. Chem., 2000, 38:
3900-3910
[5] Petrovic Z. S., Guo A., Zhuang W. J. Polym. Sci., Part A: Polym. Chem. 2000, 38:
4062-4069
[6] Javni I., Petrovic Z.S., Guo A., J. Appl. Polym. Sci., 2000, 77: 1723-1734
[7] Ahmad S., Naqvi F., Verma K.L., et al. J. Appl. Polym. Sci., 1999, 72: 1679–87
[8] Ahmad S., Ashraf S.M., Naqvi F., et al. Prog Org Coat. 2003, 47: 95–102
[9] Scott G., Polymer and the environment. Cambridge: Royal Society of Chemistry; p. 27–28, 1999
[10] Guenter, S., Rieth, R., Rowbottom, K.T., et al. Encyclopedia of Industrial Chemistry,
Wiley-VCH, sixth ed. vol. 12 pp. 269–284. 2003.
[11] Warwel, S., Klaas, M.R., J. Mol. Cat. B: Enzym., 1995, 1: 29–35
[12] Sharpless, K.B., Woodard, S.S., Finn, M.G. Pure Appl. Chem. 1983, 55: 1823–1836
[13] Vaibhav V. Goud, Anand V. Patwardhan, Narayan C. Pradhan, Bioresource Technology.
2006, 97: 1365–1371
[14] Campanella, A., Baltanas, M.A., Capel-Sanchez, M.C., et al. Green Chem. 2004, 6: 330–334
[15] Paquot, C., Hautfenne, A., Eds., In IUPAC, Applied Chemistry Division, Commission on
Oils Fats and Derivatives: Standard Methods for the Analysis of Oils, Fats and
Derivatives. Blackwell Scientific Publications: London, 1987
[16] Rangaraja, B., Harvey, A., Grulke, E.A et al. J. Am. Oil Chem. Soc. 1995, 72: 1161–1169
[17] Gan, L.H., Goh, S.H., Ooi, K.S., J. Amer. Oil Chem. Soc. 1992, 69: 347–351
[18] Okieimen, F.E., Bakare, O.I., Okieimen, C.O., J. Industrial Crops and Products. 2002,
15: 139–144
[19] Zaher, F.A., El-Mallah, M.H., El-Hefnawy, M.A., J. Am. Oil Chem. Soc. 1989, 66: 698–700
[20] Frost, A.A., Pearson, R.G., Kinetics and Mechanism, 2nd edition. John
Wiley, New York. pp. 177-84, 1961
戴宏海 杨丽庭 林博 易爱华 石光
(华南师范大学化学与环境学院 广州 510631)
摘要 研究了硫酸催化过氧乙酸氧化大豆油的动力学。利用原位生成过氧乙酸氧化大豆油合成环氧大豆油双键的转化率高而副反应环氧环的开环程度很小。得到了大豆油的氧化动力学参数,反应速率常熟为10-6 mol-1s-1,反应活化能为43.11 kJ·mol-1 ,反应的焓变、熵和自由能分别为40.63 kJ·mol-1, -208.80 kJ·mol-1 和 102.88kJ·mol-1。
关键词 动力学,环氧化,大豆油,过氧乙酸