http://www.chemistrymag.org/cji/2007/09b048pe.htm |
Nov.1,
2007 Vol.9 No.11 P.48 Copyright |
The determination of the coordination of lead(II)in the yellow river water
Guo Boshu, Jiao Xiaobao, Zhang Yi( Chemistry﹠Environment Science College of Inner Mongolia Normal University, Huhhot 010022, China )
Received Aug. 20, 2007
; Supported by the Natural Science Foundation (20467002).Abstract The complexation capacity for
Pb(II) in the Yellow River water were determinated by anodic stripping voltammetry. The
conditional stability constants and complexation capacity index were calculated. The data
showed that C(PbL) is smaller, but lgK(PbL) is large. The E (%)- pH
S curve and the peak-shaped curve were reported, and the ternary surface complexes at
liquid-solid interfaces was studied . The effect of cysteine on curves of ion exchange
ratio E(%)-pH in the interaction between Pb (II) and the surface sediment was studied. The
result showed that the ternary surface complex is the "metallike" complex.
Keywords: complexation capacity; liquid-solid interface; E(%)-pH curve; ternary
surface complexes.
1. INTRODUCTION
Usually, the concentration of organic is higher than that of heavy metal ions in natural
water. The concentration of free metal ions is controlled by dissolved organics in water.
The complexation capacity of water is its capacity for complexing with heavy metal ions
and purifying itself when heavy metal pollution occurs. It is therefore one of the
criteria for assessing water quality.
The Yellow River ranks the first in terms of silt content and
concentration among all rivers in the world. As estimated it amounts to about 200 million
tons per year. Years of observation show that the average concentration of the Yellow
River is 6.65Kg/m3 [2](Sun Jinzhu, 1994).
Sands have an immense effect on aquatic environment of the Yellow River
therefore it comes to first place among natural pollutions of the Yellow River and becomes
pollution carrier because of its enormous chelate effect on a lot of pollution drained by
human. Some kind of surface interaction in association with suspended matter such as ion
exchange, sorption, adsorption, partition, complexation or scavenging, play an important
role in the distribution and fate of trace metals in river. A method of determining
whether ternary surface complexes formed and which types these complexes should belong to
was established, the E(%)-pH curve method[5](Zhang Zhengbin, Liu Liansheng et
al, 1996).
2. EXPERIMENTAL
2.1 The complexation capacity for Pb was determined by anodic stripping
voltammetry
JP-2 oscilloscopic polarography, Chengdu Instrument Factory. Large surface (1.5m2)
mercury coated silver working electrode, platinum counter electrode and Ag/AgCl reference
electrode.
Lead storage solution were prepared by 99.999﹪high purity metals in super pure nitric acid .The concentration of
storage solution was 100mg/L, It was diluted with HCl (0.01mol/L) to the desired
concentration before use.
All of the other reagents were analytical grade.
Natural yellow River water samples were collected from Lamawan
Qingshuihe Inner Mongolia, in February, May, September 2005. Location of sampling station
in inner Mongolia at 40°N and 112°E. The samples were preserved well below-18℃。The selected potential for ASV determination of Pb: Concentrating
potential-1.20V, stripping potential-0.30V, peak potential 0.50V.
Samples used in ASV were purged with high purity nitrogen before
determination. When N2 is used to purge the air, oxygen could be driven out in
10minutes. In this experiment where we used a fast scan rate, the interference of oxygen
was diminished.
Before ASV determination,if metal
ions were added into water samples containing organic ligands, a certain length of time is
necessary. These processes of reactions are fast since 10minutes was enough for the
complexation reaction a standard metal was added in solution to the same water sample and
the determination was done after 10minutes stirring.
Dissolved organic carbon (DOC) by CrO3, NH4VO3
oxidimetry (1.94mg/L), recovery 95.6﹪.
Experiment and calculation refer to reference [4].
2.2 The curve of ion exchange ratio(%)-pH of Pb (II)
Model Z-8000 polarized zeeman atomic absorption spectrophotometer(Japan RILI; model 720 pH/ISE meter (USA ORAN); model SHA-B constant
temperature shaker (China QING DAO).
Model D/max-IIIX-ray diffractometer (Japan)
The surface sediments were taken from the section in the Yellow River
at Qingshuihe, Inner Mongolia. They were purified by the removal of trace heavy metals and
organic and were transformed into sodium ones. The purified sediments were analysed by
X-ray diffraction, infrared radiation.
The non-transition sediment is the natural sediment in the Yellow
River. As for the transition, its organic and metals on the surface removed. And it is
also a sediments transformed form the Ca-type into Na-type.
The glass were used in the experiment were immersed in the
concentration of 1: 3 HCl for two weeks, and then were immersed in Yellow River water for
another two weeks.
Surface sediments 100mg and a certain volume of natural Yellow River
water put into a series of ground glass stoppered flasks volume of 250ml, the pH of the
solution was adjusted by HCl and NH3·H2O to produce well-
distributed pH values ranging from 1 to 10. Then a certain volume of standard Pb solution
of a certain concentration and a certain volume of organic solution were added to the
above solutions in those flasks. We controlled the total volumes of organics, trace
metals, and solid particles to be just the same. The flasks were vibrated for 2.5h in a
thermostat. After filtration, the pH values of filtrate were measured for the purpose of
drawing charts, and the concentration of Pb were also determined by atomic adsorption
spectrophotometer.
3. RESULTS AND DISCUSSION
3.1 The complexation capacity (C.C) of Pb
Lead is a special ion its complexation capacity is 1.6-60 n mol·L-1,
which is lower than Cu, Zn, Cd, (Cu is 130-560 n mol·L-1, Cd is 47-99 n
mol·L-1, Zn is 48-250 n mol·L-1). But its conditional
stability constants (lgK(PbL)8.1-9.4) are higher than those of Cu, Zn, Cd, (lgK(CuL)7.7-8.0,
lgK(ZnL)7.5-8.3, lgK(CdL)7.8-8.9). It shows that some ligands are
contended little in the Yellow River, but it is able to combine with Pb solidly. It is
agreement with determination result of Pb complexation capacity of the Yellow River mouth.
In comparison with the north of the south China sea, their lgK are similar, but its C.C is
a litter larger, because the DOC is relatively small in the north of the south China sea
(the DOC of the surface water is 1.79 mg·L-1)
Complexation capacity index (C.C.I.) has a close relation with C.C and K. Through studying I, we know the complexation ratio of heavy metal with ligand and can judge the correction of C.C and K. Only when C.C and K are large, C.C determination is correct. When C.C are the same, K is large and I is large, too. When K is the same, I becomes large with C.C growing large. Although Pb’s C.C average is small (average 29 n mol·L-1), lgK average 8.9, with certain I (75.32%). As soon as I in natural water is higher than 97%, K is about 108, C.C must be at 10-5-10-4 mol·L-1, and a conclusion can be drawn that this kind of natural water may be polluted by various organic level.
Buckley and van den Berg [1] have determined and calculated organic copper with MnO2 in all depths and found that the complexation function id between 89﹪and 99.8﹪. In addition, the data is between 98.8﹪and 99.4﹪got by using DPCSV method. However, I disagree with their findings in natural water, but in fact, the complexation function of organic copper under the MnO2, method should be higher. Second, according to their determination the complexation function is too high. In that case, the concentration of organic should be more than 10-5 mol·L-1in the Atlantic Ocean. However, I think the concentration cannot be that high.
3.2 E (%)-pH of S- shaped curves
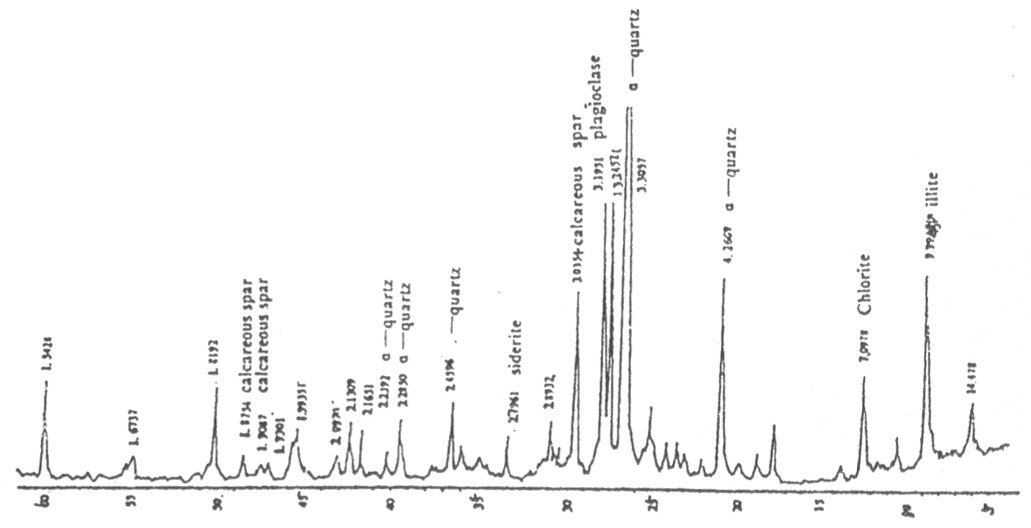
Fig.1 The spectra of powder X-ray with crystal diffraction
Table 1 Analyzing results of powder X-ray with crystal diffraction
Size(mm) |
chlorite |
illite |
quartz |
orthoclase |
plagioclase |
Calcareous |
dolomite |
hornblende |
Fe2O3 |
<0.105 |
10 |
15 |
33 |
10 |
18 |
10 |
2 |
1 |
1 |
0.125-0.105 |
10 |
20 |
34 |
13 |
13 |
4 |
2 |
1 |
3 |
0.20-0.125 |
8 |
15 |
35 |
15 |
15 |
5 |
4 |
1 |
2 |
Because
the Yellow River flows by two deserts and sediment has 35% SiO2 (see fig 1and
Table 1) which is 1:1 shape mineral and the basis structure is formed by-Si-O-Si-bond SiO4.
Because-Si-O-Si-bond is water repellent and layer charge is zero, and surface interaction
is weak .The exchange process of surface sediment is mainly formed by clay minerals which
are primarily crystalline aluminum or magnesium silicates with stacked-layer structure.
Each unit layer is in turn a dimensional array. In illite, about 1/4 Si of is replaced by
Al, which is TOT three layer. Chlorite is three-layer clay, too. Clay layer distance is a
special chemistry reaction place, which is characterized by layer exchange, layer
adsorbtion. In Yellow River water more than 80% heavy metal interacts with surface
sediments. This process is S-shape.
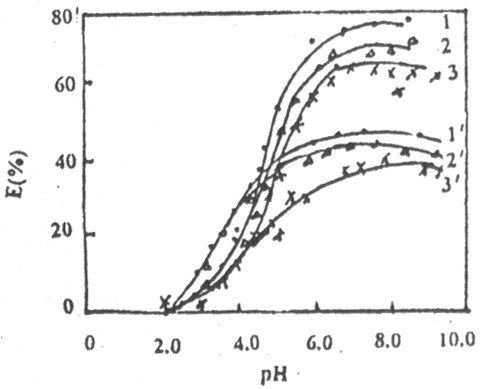
Fig.2 The relationship between exchange ratio-pH of the sediment and Pb in the Yellow
River water
1, 2, 3, (Transformed sediment)
1' 2' 3'(no
transformed sediment)
[Pb2+](mmol.·L-1): 1,
1'---2.4; 2, 2'---4.8; 3, 3'---9.6
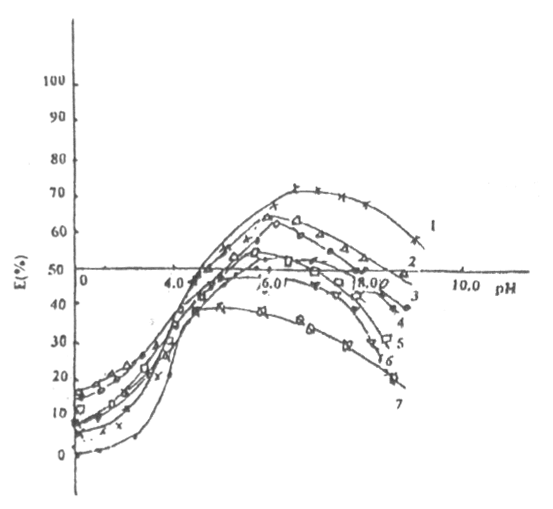
Fig.3 Effect of cystine on the curve of "E(%)-pH"(Transformed
sediment)
Cystine (mmol.·L-1): 1---0;
2---0.48; 3---2×0.48; 4---3×0.48; 5---4×0.48; 6---5×0.48; 7---10×0.48;
[Pb2+]=4.8mmol.·L-1
In Fig.2, either transformed sediment or non-transformed sediment has a maximam adsorption exchange value to Pb at pH=6.5, that is critical value in Pb of the Yellow River water. Since transformed sediment has cleared up organic carbonate and metal ion on surface, therefore it has many adsorptions active station and E (%) is greater than that of non-transformed sediment. [Pb2+] has an effect on ion exchange ratio, too. So to speak on transformed sediment, [Pb2+]=4.8mmol.·L-1, E(%)=71.5%; [Pb2+]=9.6mmol .L-1, E (%)=63.0% non-transformed sediment is in the same case.
3.3 "peak-shaped"E(%)- pH curve
From Fig.3, we can see that after cystine is added, E(%) of Pb is growing at first, but when the pH reach above 6, E (%) of reduces, depression, cystine restrains E(%). The concentration of cystine become greater, become stronger. It explained that cystine does not adsorb on the surface sediment, but to stay in the water of Yellow River.
Under pH of the Yellow River water, we determined that the lg b(oA) value is 8.37 of cystine+Pb, but the lgK(oA) of ternary surface complexes (surface sediment+cystine+Pb ) is 3.14[3] (Yang Hongwei et al,1998 Pb) For lgK(oA)<<lg b(oA), the stable chelate formed in cystine with Pb of the Yellow River water, the result is Pb from surface sediment to water, leads to E(%) degraded. Therefore, lgK(oA) and lg b(oA) determine shape of E(%)-pH curve, are important factors.
In Fig3, [Pb2+] has an effect, when pH is less than 6, the concentration of cystine has no effect to curve; pH is more than 6, E(%)-pH curve changes from s shape to peak shape. We can explain it like that, the reaction took place between cystine and Pb.

As pH increases, the reaction moves to right, the concentration of chelate became larger, so did lg b(oA), there, when it is at high pH, Pb2+ goes into the solution from above the interface and E(﹪) reduces b S-shape curve changes to"peak-shaped"curve. When [Pb2+] is constant (4.8mmol.·L-1), the concentration of cystine increases from 0 to 5×4.8mmol.·L-1, (curve 1, curve 2 …… curve 6), the E(%)-pH curve goes upward, but when pH is above 6, the E(%)-pH curve goes downwards. When the concentration of cystine increases to 4.8mmol.·L-1, (see curve 7) and when pH is about 4, the E (%)-pH curve goes downwards. So that means that when the concentration of cystine increases ten times and when the pH is about 4, ternary surface complexes decompose in advance and chelates come into existence in the Yellow River water.
3.4 Ternary surface complex
From Equation 1,we can see that the H in hydroxy on the solid surface is replaced in chelation. That show the following type of complex (ligandlike ternary complex) is impassible to come into existence.
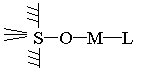
And when the pH is below 6, the metallike ternary complex comes into existence as follows.
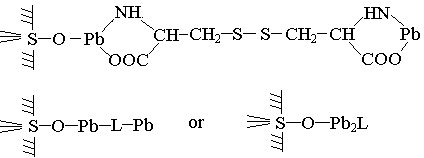
Because the molecular weight of cystine is large, R is also large, and the stability of Pb four-membered ring is relatively small, the lgK(oA) is only 3.14 and ternary surface complexes are easy to get a way from the surface of solids and get into the solution as chelate sometimes, the S-S-bond in cystine can even break, forming cysteine ternary surface complex. The reference presents a new usuful method to study ternary surface complexes at liquid-solid interface, that is the method of E(%)-pH curve. The peak-shape curve experiment using this method can also determine the type of ternary surface complex.
4 CONCLUSION
The physical chemical behavior of lead is different from that of copper, zinc and cadmium.
When lead reacts with dissolved organic ligand, their complexation capacity is small, but
stability constant is relatively high. the ternary complexes formed after the reaction
between lead and cystine on the surface is very stable. When the pH is above 6 and
concentration of cystine increases, ternary surface complexes gradually decompose; the E
(%)-pH curve becomes the peak shape curve. In addition, according to the analysis of the
experimental result, the ternary surface complex in this experiment is the metallike
ternary surface complex.
[1] Buckley.P.J.M. and van den Berg.C.M.G.1986, Copper Complexation profiles in Atlantic Ocean. Marine Chemistry, 19: 281-296.
[2] Sun Jinzhu,1994,The Early Warning and Dredge Countermeasure of Inner Mongolia Life and Relation to the Environment. Inner Mongolia peoples press, Huhhot, P26-30.
[3] Yang Hongwei, Jiao Xiaobao, Guo Boshu et al,1998, The curve of Ion Exchange Ratio(%)-pH of the Interaction between Suspended Particles with Cd (Ⅱ) in the Yellow River. Journal of Environmental Sciences.10 (2):252-256.
[4] Yang YuYing, Wu Di, Hong Xia, Guo BoShu, 1999. Determination of Complexation Capacity of trace Metal-Organic in natural Water, Journal of Environmental Sciences, 11(1):124-128.
[5] Zhang Zhengbin, Liu Liansheng, Zhao Hongbin, Fu Youjun and Wu Zhijian, 1996. Studies of Ternary Surface Complexes at Liquid-Solid Interfaces in Seawater by the E(%)-pH curve Method. Journal of Colloid and Interface Science. 182:158-165. 黄河水中铅(Ⅱ)配位作用的测定
郭博书 焦小宝 张毅
(内蒙古师范大学化学与环境科学学院,内蒙古呼和浩特010022)
摘要 用阳极溶出伏安法测定了黄河水体中铅(II)的络合容量,计算了条件稳定常数和络合容量指数,数据表明:C.C(PbL)较小(16-60nmol·L-1)但lgK(PbL) 却较大(8.1-9.4)。报道了E(﹪)-pH S形曲线和峰形曲线,研究了液-固界面三元表层络合物。也研究了半胱氨酸对Pb(Ⅱ)和表层沉积物之间相互作用的E(﹪)-pH曲线上离子交换率的影响。结果表明三元表层络合物为“类金属”络合物。
关键词 络合容量;液-固界面作用;E(﹪)-pH曲线;三元表层络合物