http://www.chemistrymag.org/cji/2007/09b049ne.htm |
Nov.1,
2007 Vol.9 No.11 P.49 Copyright |
Synthesis and characterization of N, N'-substituted bis (2, 5-dimethyl-3, 4-diacetyl) pyrrole derivatives and N, N'-substituted bispyrrolo [3, 4-d] pyridazine derivatives
Zhang
Shuwen, Hao Pengpeng, Wei Baojun
(College of Chemistry and Environmental
Science, Hebei University, Baoding, 071002, China)
Received Sep. 26, 2007.
Abstract N, N'-Substituted bis (2,
5-dimethyl-3, 4-diacetyl) pyrrole derivatives 2a-2e were synthesized via Paal-Knorr
reaction of 3, 4-diacetyl-2, 5-hexanedione with diamine in the solvent of acetic acid and
ethanol. N, N'-substituted bispyrrolo [3, 4-d] pyridazine derivatives 3a-3e were
synthesized by the reaction of pyrroles 2a-2e and excess hydrazine in the solvent.
The structures of products were characterized by IR, 1H NMR spectra and
elemental analysis.
Keyword N, N'-Substituted bis (2, 5-dimethyl-3, 4-diacetyl) pyrrole, 3,
4-diacetyl-2, 5 -hexanedione, N, N'-substituted bispyrrolo [3, 4-d] pyridazine, synthesis,
Paal-Knorr reaction.
1 INTRODUCTION
Pyrrole and its derivatives are ubiquitous among
naturally occurring organic compounds. These compounds can have intrinsic
biological activity and also constitute the structural feature of many biologically active
compounds. They are commonly found as structural motifs in bioactive molecules such as
porphyrins, alkaloids and coenzymes [1]. The authors have reported
polysubstituted bispyrrole compounds [2] before. In this paper N,
N'-Substituted bis (2, 5-dimethyl-3, 4-diacetyl) pyrrole derivatives, and the
diacetylpyrrole derivatives exposed to excess hydrazine hydrates giving the
polysubstituted pyrrolopyridazines were reported. The Pyrrolopyridazine compounds were
used to the treatment of psychiatric and mood disorders[3] such as, for
example, schizophrenia, anxiety, depression, bipolar disorders and panic, as well as to
the treatment of pain Parkinson's disease, cognitive dysfunction epilepsy, circadian rhythm and
sleep disorders-such as shift-work induced sleep disorder and jet-lag ,drug addition, drug
abuse, drug withdrawal and other diseases. They also display high-affinity binding
particularly selective binding to the subunit of voltage gated calcium channel [3-5].
In this paper five N, N'-Substituted bis (2, 5-dimethyl-3, 4-diacetyl) pyrrole
derivatives and five polysubstituted pyrrolopyridazines are synthesized (Scheme 1);
the structures of products were characterized by IR, 1H NMR spectra and
elemental analysis.
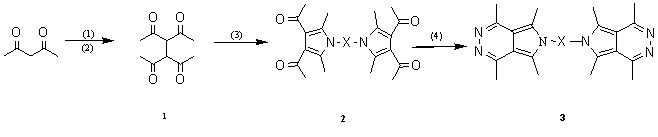
(1) THF / EtONa or THF / t-BuOK; (2) Br2; (3) H2N-X-NH2/ EtOH: HOAC = 4: 3; (4) NH2NH2
(80%)
2a ,3a: X = ( CH2 )2 NH ( CH2 ) 2 ; 2b
,3b: X = ( CH2 )2 NH ( CH2 )2 NH ( CH2
)2 ; 2c,3c:X = ( CH2 )4 ;2d,3d: X = C6H4SO2NHC6H4
2e,3e : X =C7H6C7H6
Scheme 1
2 EXPERIMENTS
2.1 Materials and instruments
The new compounds were characterized by data of 1H NMR, IR and element analyses and were described in the experimental section. IR spectra were recorded on a HITACHI 260-50 spectrometer (KBr). 1H NMR spectra were measured on an AVAVCE-400 spectrometer using TMS as internal standard and CDCl3 or MeOD as solvent. Elemental analysis measured on a HERAEUS (CHN, Rapid) analyzer. M.p. was measured on a XT-4 melting point instrument (thermometer uncorrected).
All of the reagents and solvents were AR grade. Absolute alcohol was dehydrated by distillation after sodium addition.
2.2 Preparation of 3, 4-diacetyl-2, 5- hexanedione (1)
2, 4-Pentanedione 5.0 g, (0.05 mol) was added dropwise to stirred slurry of sodium ethylate 2.9 g, (0.05 mol) and THF 50 mL. Br2 4.0 g, (0.025 mol) was then added dropwise and stirred for 2 h. Most of THF was then removed and the residue was cooled and filtered. The solid was washed with water and crystallized from acetone. After crystallization from acetone it had m. p. 188-189 oC (lit. [6] 189-190oC).
2.3 Preparation of N, N'-Substituted Bis (2, 5-dimethyl-3, 4- diacetyl) pyrrole derivatives
The diamine 10 mmol was added to stirred slurry of diacetylhexanedione 3.96 g, (20 mmol) and ethanol 9 mL and acetic acid 12 mL. The mixture was heated under reflux several hours. The procedure was monitored by TLC. After completion of the reaction, the mixture was cooled to room temperature and purified by various methods.
2.3.1 Preparation of 2, 2'-Bis (2, 5-dimethyl-3, 4- diacetylpyrrol-1-yl) diethylamine (2a)
Diamine was diethylenetriamine. The water was added to the mixture, and then the resulting pyrrole was isolated by filtration, recrystallized from water to give yellow solid 3.10 g, the yield was 72.5%, m. p. 200oC. 1H NMR (CDCl3, 400 MHz) d: 3.91 (t, J = 6.8 Hz, 4H, 2PyN-CH2), 2.84 (t, J= 6.8 Hz, 4H, 2N-CH2), 2.37 (s, 12H, 2×2Py-CH3), 2.40 (s, 12H, 2×2COCH3). IR (KBr) n: 2975, 1665, 1395, 1355, 1165 cm-1. Anal. Calcd for C24H33O4N3: C 67.42, H 7.78, N 9.83; found C 67.19, H 7.81, N 9.86.
2.3.2 Preparation of 2, 2'-Bis [(2, 5-dimethyl-3, 4-diacetylpyrrol-1-yl) ethyl] ethylenediamine (2b)
Diamine was triethylenetetramine. The solid was isolated by filtration after dilution of the mixture with ethyl acetate. Recrystallization from ethyl acetate gave yellow solid 3.24 g; the yield was 69.5%, m. p.130oC. 1H NMR (CDCl3, 400 MHz) d: 3.99 (t, J = 6.4 Hz, 4H, 2PyN-CH2), 3.27 (t, J = 6.4 Hz, 4H, 2N-CH2), 3.18 (t, J = 6.4 Hz, 4H, 2N-CH2), 2.41 (s, 12H, 2×2Py-CH3), 2.30 (s, 12H, 2×2COCH3). IR (KBr) n: 3310, 2895, 1661, 1396, 1358, 1421, 1164 cm-1. Anal. calcd for C26H38O4N4: C 66.36, H 8.14, N 11.91; found C 66.17, H 8.17, N 11.87.
2.3.3 Preparation of 1, 4 -Bis (2, 5-dimethyl-3, 4-diacetylpyrrol-1-yl) butane (2c)
Diamine was butanediamine. The solid was isolated by filtration after dilution with ether. Crystallization from ethanol gave yellow crystal 3.14 g, the yield was 76.1%, m. p. 152-154oC. 1H NMR (CDCl3, 400 MHz)d: 4.29 (t, J = 7.2 Hz, 4H, 2PyN-CH2), 2.39 (s, 12H, 2×2COCH3), 2.34 (s, 12H, 2×2Py-CH3), 1.34 (t, J=7.2 Hz, 4H, 2CH2). IR (KBr) n: 2943, 1665, 1639, 1394, 1357, 1158 cm-1. Anal. Calcd for C24H32O4N2: C 69.88, H 7.82, N 6.79; found C 69.69, H 7.85, N 6.81.
2.3.4 Preparation of 4, 4'-Bis (2, 5-dimethyl-3, 4-diacetylpyrrol-1-yl)-N-phenylbenzenesulfon -amide (2d)
Diamine was 4, 4’-Diaminobenzenesulfonanilide. The water was added to the mixture, and then the resulting pyrrole was isolated by filtration. Recrystallized from acetone-water gave yellow crystal 4.85 g, the yield was 82.6%, m. p. 143-144oC. 1H NMR (CDCl3, 400 MHz) d: 8.04 (d, J = 8.8 Hz, 2H, 2ArH), 7.37 (d, J=6.0 Hz , 4H, 4ArH), 7.12 (d, J = 8.4Hz, 2H, 2ArH), 2.46 (s, 6H, 2Py-CH3), 2.45 (s, 6H, 2Py-CH3), 2.08 (s, 6H, 2COCH3), 2.06 (s, 6H, 2COCH3). IR (KBr) n: 3035, 1662, 1558, 1520, 1395, 1346, 856 cm-1. Anal. Calcd for C32H33O6N3S: C 65.40, H 5.66, N 7.15; found C 65.16, H 5.68, N 7.17.
2.3.5 Preparation of 3, 3'-dimethyl-4, 4'-Bis (2, 5-dimethyl-3, 4-diacetylpyrrol-1-yl) diphenyl (2e)
Diamine was 3,3'-bismethyl-4,4'-bisamidobiphenyl.The solution was cooled and filtered .The solid was crystallized from trichloromethane-ethanol to give pale yellow crystal 4.50 g, the yield was 84%, m.p.277-279oC. 1H NMR (CDCl3, 400 MHz)d: 7.66 (s, 2H, 2ArH), 7.62 (d, J = 8.4 Hz, 2H, 2ArH). 7.24 (d, J = 8.0 Hz, 2H, 2ArH), 2.49 (s, 12H, 2×2COCH3), 2.12 (s, 12H, 2×2Py-CH3), 2.10 (s, 6H, 2Ar-CH3). IR (KBr) n: 3046, 2945, 1668, 1559, 1500, 1392, 1358, 838 cm-1. Anal. Calcd for C34H36O4N2: C 76.09, H 6.76, N 5.22; found C 75.85, H 6.78, N 5.20.
2.4 Preparation of N, N'-substituted pyrrolo [3, 4-d] pyridazine derivatives
2.4.1 Preparation of 2, 2'-Bis (1, 4, 5, 7-tetramethyl-pyrrolo [3, 4-d] pyridazin-6-yl) diethylamine (3a)
Hydrazine hydrate (80% solution) 0.7 mL was added to stirred slurry of pyrrole (2a) 1.0 g and ethanol 40 mL-water 6 mL. The mixture was heated under reflux for 0.5 h. Then cooled and diluted with water. The solid was filtered off, washed, and dried. Crystallization from methanol-water gave pale yellow solid 1.00g, the yield was 92.6%. 1H NMR (MeOD, 400 MHz) d: 4.40 (t, J = 6.3 Hz, 4H, 2PyrroloN-CH2), 3.47 (t, J= 6.6 Hz, 4H, 2N-CH2), 2.73 (s, 12H, 2×2Pyridazine-CH3), 2.66 (s, 12H, 2×2Pyrrole-CH3). IR (KBr) n: 2954, 1650, 1633, 1550, 1461, 1077 cm-1. Anal. Calcd for C24H33N7: C 68.70, H 7.93, N 23.37; found C 68.49, H 7.91, N 23.45.
2.4.2 Preparation of 2,2’-Bis[(1,4,5,7-tetramethyl-pyrrolo[3,4-d]pyridazin-6-yl)ethyl]ethylene -diamine (3b)
To a solution of pyrrole (2b) 1.0 g and ethanol 30 mL was added hydrazine hydrate (80% solution) 0.7 mL. The mixture was heated under reflux for 1.5 h, after cooling to r.t. The mixture was isolated by filtration after dilution with water. The solid was crystallized from methanol-water in white solid 0.90g, the yield was 91.8%. 1H NMR (MeOD, 400 MHz) δ: 4.28 (t, J = 6.6Hz, 4H, 2 PyrroloN-CH2), 3.09 (t, J=6.6 Hz, 4H, 2N-CH2), 2.88 (t, J = 6.6 Hz, 4H, 2N-CH2), 2.69 (s, 12H, 2×2Pyrrole-CH3), 2.66 (s, 12H, 2×2Pyridazine-CH3). IR (KBr) n: 2955, 1667, 1644, 1550, 1450, 1122 cm-1. Anal. Calcd for C26H38N8: C 67.50, H 8.28, N 24.22; found C 67.29, H 8.31, N 24.14.
2.4.3 Preparation of 1, 4'-Bis (1, 4, 5, 7-tetramethyl-pyrrolo [3, 4-d] pyridazin-6-yl) butane (3c)
The mixture of pyrrole (2c) 1.0 g and hydrazine hydrate (80% solution) 0.7 mL, in the solution of ethanol 30 mL was heated under reflux for 1.0 h. Gave pyrrolopyridazine as a white solid 0.96 g, The yield was 97.9%. 1H NMR (MeOD, 400 MHz) d: 4.28 (t, J = 7.3 Hz, 4H, 2PyN-CH2), 2.70 (s, 12H, 2×2Pyridazine-CH3), 2.68 (s, 12H, 2×2Pyrrole-CH3), 1.83 (t, J=7.3 Hz, 4H, 2CH2). IR (KBr) n: 2924, 1667, 1656, 1467, 1050 cm-1. Anal. Calcd for C24H32N6: C 71.25, H 7.97, N 20.77; found C 71.01, H 8.00, N 20.85.
2.4.4 Preparation of 2, 2'-Bis (1, 4, 5, 7-tetramethyl-pyrrolo [3, 4-d] pyridazin-6-yl) - N-phenylbenzenesulfon-amide (3d)
The mixture of pyrrole (2d) 0.5 g and hydrazine hydrate (80% solution ) 0.5 mL, in the solution of ethanol 20 mL-acetone10 mL was heated under reflux for 0.5 h. Gave Pyrrolopyridazine as a white solid 0.17 g, The yield was 86.3%. 1H NMR (MeOD, 400 MHz) d: 8.15 (d, J = 8.5 Hz, 2H, 2ArH-SO2 ), 7.55 (d, J=8.4 Hz, 2H, 2ArH-Pyrrolo), 7.39 (d, J = 8.7 Hz, 2H, 2ArH-Pyrrolo), 7.20 (d, J=8.7 Hz, 2H, 2ArH-NH), 2.85 (s, 6H, 2 Pyridazine-CH3), 2.83 (s, 6H, 2 Pyridazine-CH3), 2.44 (s, 6H, 2Pyrrole-CH3), 2.43 (s, 6H, 2Pyrrole-CH3). IR (KBr) n: 3034, 2931, 1656, 1561, 1333, 1167, 860, 833 cm-1. Anal. Calcd for C32H33O2N7S: C 66.30, H 5.74, N 16.91; found C 66.12, H 5.76, N 16.85.
2.4.5 Preparation of 3, 3'-dimethyl-4, 4'-Bis (1, 4, 5, 7-tetramethyl-pyrrolo [3, 4-d] pyridazin-6-yl) diphenyl (3e)
The pyrrole (2e) 0.7 g and hydrazine hydrate (80% solution) 0.4 mL, in the solution of boiling ethanol 10 mL- trichloromethane 10 mL, reacted to give pyrrolopyridazine as a white solid 0.65g, The yield was 94.2%. 1H NMR (CDCl3, 400 MHz) d: 7.75 (s, 2H, 2ArH), 7.71 (d, J = 8.0 Hz, 2H, 2ArH). 7.30 (d, J = 8.8 Hz, 2H, 2ArH), 2.83 (s, 12H, 2×2Pyridazine-CH3), 2.45 (s, 12H, 2×2Pyrrole-CH3), 2.00 (s, 6H, 2Ar-CH3). IR (KBr) n: 3045, 2920, 1656, 1583, 1466, 867, 802 cm-1. Anal. Calcd for C34H36N6: C 77.24, H 6.86, N 15.90; found C 77.01, H 6.88, N 15.95.
3 RESULTS AND DISCUSSION
The authors have synthesized five pyrrole derivatives (2a-2e) in 70-80% yields and
five pyrrolopyridazine derivatives (3a-3e) in mostly above 90% yields conveniently.
The synthesis of pyrrole derivatives (2a-2e)
was simple but much more time consumed for reaction. We attempted to adjust the proportion
of ethanol and acetic acid to accelerate the reaction rate but not fulfilled.
There are many
articles [7-10]
4 CONCLUSIONS
In this paper, five N, N'-Substituted bis (2, 5-dimethyl-3, 4-diacetyl) pyrrole derivatives were synthesized via Paal-Knorr reaction and five N, N'-substituted pyrrolo [3, 4-d] pyridazine derivatives were synthesized by the reaction of pyrroles with excess hydrazine in the solvent. All of them are new compounds .The structures of products were characterized by IR, 1H NMR spectra and elemental analysis.
REFERENCES
[1] Franck B. Nonn A. Angew. Chem., Int. Ed. Engl., 1995, 43: 1975.
[2] Li Z Y, Zhang S W, Cheng Z H, et al. Chemical Journal on Internet (Guo Ji Wang Shang Hua Xue Xue Bao ), 2006, 8 (12): 75.
[3] Burke A N, WO2004006836, 2004-01-22.
[4] Stearns B A. Anker N. Arruda J M. et al. Bio. Med. Chem. Lett., 2004, 14: 1295-1298.
[5] Hu T. Stearns B A. Campbell B T. et al. Bio. Med. Chem. Lett., 2004, 14: 2031-2034.
[6] Mosby W L. J. Chem. Soc., 1957, 9: 3997-4003.
[7] Hernandez T. Arques J S. Dawes H M., et al. J. Chem. Soc., Perkin Trans.1, 1985, 1: 899-902.
[8] Rips R. BUU-HOÏN P. J. Org. Chem., 1959, 24 (4):551-554.
[9] Rodriguez F F. J. Chem. Res. Miniprint., 1987, 11:2901-2914.
[10] Kreher R, Vogt G. Angew. Chem., 1970, 82 (23): 958. N,N'-取代双(2,5-二甲基-3,4-二乙酰基)吡咯衍生物及N,N'-取代双吡咯并[3,4-d]哒嗪衍生物的合成及表征
张书文,郝鹏鹏,魏保君
(河北大学化学与环境科学学院;中国河北保定 071002)
摘要 本文以3,4-二乙酰基-2,5-己二酮和二元伯胺类化合物为原料通过 Paal-Knorr 反应,以乙酸和乙醇为溶剂合成了N,N'-取代双(2,5 -二甲基-3, 4-二乙酰基)吡咯衍生物,将此吡咯衍生物再与过量的水合肼反应进一步合成了N,N'-取代二吡咯并[3,4-d]哒嗪衍生物。并通过红外光谱、核磁共振氢谱及元素分析对化合物进行了表征.
关键词 N,N'-取代双(2,5-二甲基-3,4-二乙酰基)吡咯,3,4-二乙酰基己二酮, N,N'-取代双吡咯并[3,4-d]哒嗪,合成, Paal-Knorr反应