http://www.chemistrymag.org/cji/2007/09b050ne.htm |
Nov.1,
2007 Vol.9 No.11 P.50 Copyright |
Isolation of 2-(1',2',3',4'-tetrahydroxybutyl)-6-(2",3",4"-trihydroxybutyl)-Pyrazine from Yunnan flue-cured tobacco and its syntheses
Chen Yongkuan1, Kong Ningchuan1,
Li Cong2, Wang Hanqing3
(1 Yunnan Reascend Tobacco Technology Co. LTD., Kunming, 650106,China; 2Department
of Application Chemistry, Yunnan University, Kunming, 650091, China; 3State Key
Laboratory of OSSO, Lanzhou Institute of Chemistry Physics, Chinese Academy of Sciences,
Lanzhou, 730000, China)
Received Aug. 20, 2007.
Abstract A C12-polyhydroxy
derivative of 1,4-diazine isolated from Yunnan flue-cured tobacco was identified as
2-(1',2',3',4'-tetrahydroxybutyl)-6-(2",3",4"-trihydroxybutyl)-pyrazine.
Its structure was determined by the spectral data and synthetic method.
Keywords flue-cured tobacco; polyhydroxy pyrazine derivative; isolation; synthesis
A large number of the volatile flavor constituents of tobacco have been thought to be
relative to pyrolysis of precursors during heating and burning of tobacco. In contrast
with many examinations of precursors, reports having been published mainly discussed
nor-carotenoids and flavonoids. A C13-nor-carotenoid glucoside and a C11-nor-carotenoid
glucoside from flue-cured tobacco had been isolated and identified[1,2].
However, very few reports on the precursors of polyhydroxy derivatives of pyrazines have
been published. In this paper, the authors first report the isolation and identification
of a polyhydroxy pyrazine from Yunnan flue-cured tobacco as
2-(1',2',3',4'-tetrahydroxybutyl)-6-(2",3",4"-trihydroxybutyl)-pyrazine.
Its structure was identified by the spectral data and synthetic method.
The isolation procedure for polyhydroxy pyrazine I was illustrated in
Fig.1. The isolation procedure was improved upon the method described in the literature1.
The MeOH No.3 fraction (960mg) was obtained from flue-cured tobacco(3kg). Further, the
MeOH No.3 fraction was re-crystallized. After successively crystallization twice,
polyhydroxy pyrazine I was obtained as a white powder sample(18mg) with [a]20 D=-94.6 (1.15×10-3,
H2O) and mp 380oC(decomposing). The molecular weight of polyhydroxy pyrazine I was
determined to be 304 by FAB mass spectrometry. The strong absorption at 275nm in the UV
spectra was due to the conjugated aromatic ring, and the stretching vibration broad band
at 3308cm-1 in the infrared spectra showed that there associated with hydroxyl
group in the structure. The 1HNMR with D2O and DOCD3 as
solvent at 20oC showed that the compound has 13 non-active protons, and two of them
are in low field, indicated that the protons attached to the aromatic ring. When DMSO and
Eu(dpm) 3 were substituted D2O and DOCD3 as solvent at
100oC, 7 active protons clearly showed up. It is indicated that there
are 7 hydroxy protons in the compound. The 13CNMR of DEPT demonstrated only 10
carbons with missing 2 carbons in low field. This implicated that the 12 carbons in this
compound were different: 3 of them were secondary carbons (CH2), 7 tertiary
(CH) and 2 quarternary (C). Furthermore, the two tertiary carbons in low field for the 3-C
and 5-C, together with missing two for the 2-C and 6-C, strongly suggested that the title
compound was C12H20N2O7 with a ring and double
bond 4. The structure of the compound (Fig.2) has been determined by the 1H-1H
COSY, 13C-1H COSY and its chemical shifts listed in Table 1. Among
them, the long range heteronuclear correlation between 1'-H and 2-C and between 1'-H and
3-C indicated the 1'-C bonding to 2-C directly. Similarly, the long range heteronuclear
correlation between 1"-H and 6-C and between 1"-H and 5-C indicated the 6-C
bonding to 1"-C directly. The HMBC point of 5-C with 3-H, and of 3-C with 5-H,
clearly showed the 2,6-bissubstituted pyrazine.
To confirm the above structural elucidation, polyhydroxy pyrazine II
was prepared by the Maillard reaction[3] (Fig.3). D-glucose(1.8g), ammonium
formate(4.5g) and 10 ml H2O were mixed. After the mixture was refluxed at 100oC for 6h,
it was concentrated under reduced pressure in a water bath temperature not exceeding 45oC. And
MeOH (10ml) was added to the concentration. The filtrate was cooled down to 0oC and
placed for 24h. A buff crystal can be obtained. After successively crystallization three
times with water, polyhydroxy pyrazine II was obtained as a white powder sample. The 1HNMR,
13CNMR and IR spectra of the prepared polyhydroxy pyrazine II were identical
with those of polyhydroxy pyrazine I obtained from Yunnan flue-cured tobacco. The absolute
configuration of the polyhydroxy pyrazine ,however, could not be determined owing to the
small amount of the sample. Up to now, there has been no clear explanation for the
formation of tobacco polyhydroxy pyrazines. It needs our further researches whether or not
the polyhydroxy pyrazines are considered to be a precursor of volatile pyrazines
constituents.
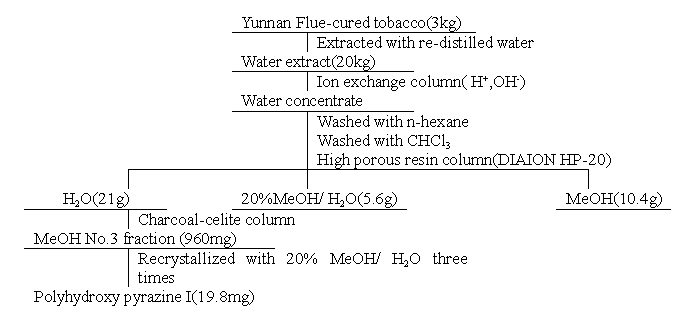
Fig.1 Isolation Procedure of Polyhydroxy
Pyrazine I
Table 1 The assignment of C, H of th
e title compound isolated from Yunnan tobaccoposition |
d13C | d1H | position |
d13C | d1H |
1' |
72.2 |
5.14,5.15(d) |
5 |
144.3 |
8.20(s) |
2' |
74.2 |
3.82(m) |
6 |
154.8 |
|
3' |
71.8 |
3.78(m) |
1" |
38.5 |
2.95(m),3.16(m) |
4' |
63.8 |
3.82(m),3.62(m) |
2" |
72.0 |
4.00(m) |
2 |
156.8 |
3" |
75.1 |
3.62(m) |
|
3 |
141.1 |
8.40(s) |
4" |
63.3 |
3.78(m),3.63(m) |
s=single; d=double; m=multiple
EXPERIMENTAL
Chemicals. The following compounds were obtained commercially: D-glucose, ammonium
formate, n-hexane , methanol(Beijing chemical plants). The solvents were distilled before
use.
Instrumental analysis. Mass spectra was obtained with VG AutoSpec-3000. Infrared
spectra was obtained with Bio-Rad FtS-135. Specific rotation was measured with a JASCO
DIN-370. 1HNMR and 13CNMR spectra were obtained Bruke AM400 and
DRX-500. Melting point was obtained with a XRC-1. The chemical shifts are as ppm field
from Me4Si as internal standard.
Isolation of polyhydroxy pyrazine I. The isolation procedure was shown in Fig.1.
polyhydroxy pyrazine I was obtained as white powder crystals. [a]20 D=-94.6 (1.15×10-3, H2O)
and mp 380oC(decomposing). IR(cm-1): 3308. MS(m/z): 304. UV(nm):
375. 1HNMR(δ): 2.94(m), 3.15(m), 3.62(m),
3.63(m), 3.65(m), 3.76(m), 3.79(m), 3.81(m), 3.83(m), 4.02(m), 5.15, 5.16(d), 8.22(s) ,
8.41(s). 13CNMR(δ): 38.5, 63.3, 63.9,
71.8, 72.0, 72.2, 72.3, 75.2, 141.1, 144.3, 154.8, 156.9.
Synthesis of polyhydroxy pyrazine II. The possible synthetic processing was shown
in Fig.3. Polyhydroxy pyrazine II was obtained as white powder crystals. [a]20 D=-94.8 (1.15×10-3,
H2O) and mp 380℃(decomposing). IR(cm-1):
3307. MS(m/z): 304. UV(nm): 375. 1HNMR(δ):
2.95(m), 3.16(m), 3.61(m), 3.62(m), 3.63(m), 3.78(m), 3.79(m), 3.82(m), 3.83(m), 4.00(m),
5.14, 5.15(d), 8.20(s), 8.40(s). 13CNMR(δ):
38.7, 63.5, 64.0, 72.1, 72.2, 72.4, 74.4, 75.4, 141.2, 144.4, 155.0, 157.0.
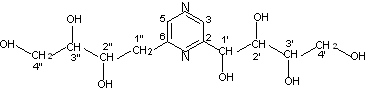
Fig.2 The structure of the title compound
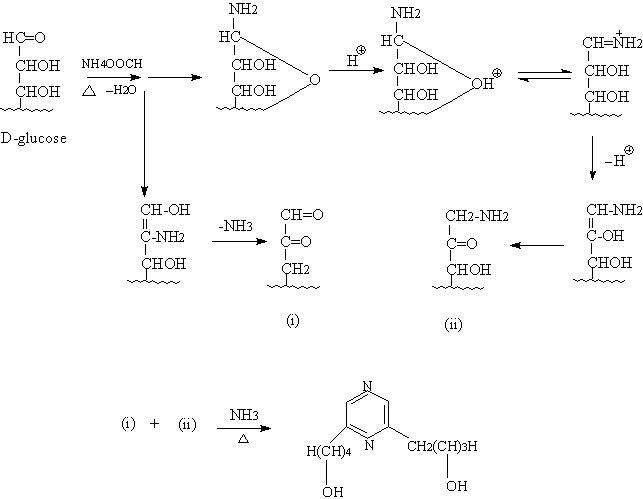
Fig.3 The possible synthetic processing of the title compound
REFERENCES
[1]Hisashi Kodama, Takane Fujimori, Kunio Kato. Agric.Biol.Chem.,1982,46(5):
1409-1411.
[2] Hisashi Kodama, Takane Fujimori, Kunio Kato. Agric.Biol.Chem.,1982,45(3): 941-943.
[3]Li H, Li PW, Yang ZH. Chemistry of heterocyclic compounds in flavors and aroms. 1st
edition. Beijing: China Light Industry Press, 1992(in Chinese).
云南烤烟中2-(1',2',3',4'-四羟基丁基)-6-(2",3",4"-三羟基丁基)吡嗪的分离与合成
陈永宽1,孔宁川1,李聪2,汪汉卿3
(1 云南瑞升烟草技术(集团)有限公司,昆明650106;2云南大学应用化学系,昆明650091;3中科院兰州化学物理研究所OSSO国家重点实验室,兰州730000)
摘要 从云南烤烟中提取分离得到了一个具有C12结构的1,4-吡嗪的多羟基衍生化合物,并采用光谱技术鉴定其为2-(1',2',3',4'-四羟基丁基)-6-(2",3",4"-三羟基丁基)吡嗪。同时,采用合成技术对其结构进行了确认。
关键词 烤烟;多羟基吡嗪衍生物;分离;合成