http://www.chemistrymag.org/cji/2007/09c052pe.htm |
Dec.1,
2007 Vol.9 No.12 P.52 Copyright |
Bo Lili, Yang
Wu, Xue Qunji, Gao Jinzhang
(College of Chemistry and Chemical Engineering, Academy of West-China Resource
Applications, Northwest Normal University, Lanzhou 730070, China)
Received Oct.24, 2007.
Abstract Ag/polyvinyl alcohol colloids were prepared by in situ reduction method,
in which silver nitrate, sodium borohydride and polyvinyl alcohol(PVA) acted as precursor,
reductant and stabilizer respectively. The synthesized colloids have potent antibacterial
activity toward both Gram-positive and Gram-negative bacteria. Further studies have
demonstrated that the distribution of Ag nanoparticles protected with the polymer chain of
polyvinyl alcohol was uniform. UV-Vis spectroscopy, X-ray diffraction (XRD), transmission
electron microscopy (TEM) and atom force microscopy (AFM) techniques were employed to
characterize the structure and property of the nanocomposites.
Keywords Ag nanocomposite colloids, antimicrobial, polyvinyl alcohol, chemical
synthesis
1. INTRODUCTION
Silver and its compounds have been known
to have extraordinary bacterium-inhibitory and bactericidal properties many a day. The
silver ion exhibits broad-spectrum biocidal activity toward many different bacteria,
fungi, and viruses
[1,2] and is believed to be the active component in silver-based
antimicrobials. Silver has low toxicity toward mammalian cells[3,4] and does
not easily provoke microbial resistance[5,6]. Because of these properties, silver-based materials have been
widely studied in the past decades. Nanometric silver particles(nano-Ag) prepared by a
variety of synthetic methods have been shown to be effective antimicrobial agents [7-11]. Recently, Chi-ming Che's group demonstrated that nano-Ag synthesized by sodium
borohydride reduction exhibited cytoprotective activities toward human immunodeficiency
virus-1 infected cells [12].
Many methods, such as chemical reduction, polyol process and
radiolytic method [13-16],
have been empolyed for the synthesis of silver nanoparticles. These methods are expected
to result in a narrow particle size distribution and particles of uniform shape. Since the
metal colloids tend to coagulate, they are usually unstable and difficult to be used. As a
result, their antibacterial activities are poor. This problem can be greatly overcome by
embedding or encapsulating the metal nanoparticles with polymer materials [17].
Silver nanoparticles protected by polymers, such as PVA[18,19],
poly(vinylpyrrolidone)(PVP)[20-22], polystyrene(PS)[23] or
polymethylmethacrylate (PMMA) [24], have been extensively reported. PVA could be considered as
a good host material for metal, due to its excellent thermo-stability and chemical
resistance [25,26]. In addition, owing to its water solubility, the silver
nanoparticles can be easily prepared in aqueous medium and the preparation is virtually
non-toxic.
Many techniques
have been developed to prepare the metal/polymer nanocomposites, such as electrochemical
reduction and some complicated physical techniques[27] including sputtering[28-30]
and plasma deposition[31,32]. However these techniques are usually
time-exhausting, costly and complicated. Hence, a simpler method to incorporate silver
into polymers is desirable. The chemical reduction way is a preferred one to synthesize
the metal/polymer nanocomposites, because of its convenient operation and simple
equipment.
In this paper, an easy synthetic route for silver nanoparticles
embedded in polyvinyl alcohol is described. Silver nitrate was reduced by sodium
borohydride in aqueous PVA solution. The results show that prepared Ag/PVA colloids have
much higher long-lasting antibacterial activity toward both gram-positive and
gram-negative bacteria than nano-Ag without the stabilization of PVA.
2.1. Materials
All of the chemicals and reagents used were of analytical grade. Distilled water was used throughout the work. Polyvinyl alcohol (MW about 1600) and sodium borohydride were purchased from Sanpu Chemical Engineering Ltd. Corp.(Shanghai, China). AgNO3 was obtained from Tianxin Fine Chemical Engineering Ltd. Corp.(Tianjin, China).
2.2. Preparation of Ag nanoparticles with PVA as stablizer
In a typical experiment, 0.2000 g PVA was dissolved in 25.00 mL distilled water at 80oC by vigorously stirring to form homogeneous solution. An aqueous solution 20.00mL of 0.01mol.L-1 AgNO3 was prepared and added to the above PVA solution maintaining the weight ratio of AgNO3 to PVA approximately at 1:10. This mixture solution was then allowed to stir in a Schlenk flask for about 10 min. And then the solution was purged with nitrogen. The fresh dilute aqueous solution of 5×10-3 mol.L-1 15.00mL sodium borohydride was prepared and introduced to the PVA/AgNO3 solution drop by drop. The color of the solution started to change from yellow to golden yellow along with reduction of Ag+ to Ag0. Stirring was continued for another 15 min under inert atmosphere at room temperature. Nano-Ag without the stabilization of PVA was similarly prepared in the absence of PVA.
2.3. Antibacterial activities
2.3.1.Bacterial culture
E. coli (ATCC25922), S. aureus (ATCC25923) and P. aeruginosa(ATCC27853) were grown at 35oC and maintained on LB plates (Luria-Bertani broth, Lennox modification, with 1.5% agar) for 18-20 h. C. albicans(ATCC90028) was grown at 28oC .
2.3.2 Disk-diffusion test of Ag/PVA nanocomposite on planar supports
A modified Kirby Bauer disk diffusion technique as described previously by Melaiye et al [33] was used to probe the bactericidal effect of the Ag /PVA nanocomposites against E.coli, S. aureus and P. aeruginosa. Identically sized filter papers were coated with the Ag/PVA composite solution in ethanol and then dried. These filter papers were then placed on bacteria-inoculated agar plates. The agar plates were then inverted and incubated at 35oC for 20 h. The zone of inhibition (ZoI), where no visible bacterial colonies formed, was measured using a digital caliper.
For C. albicans(ATCC90028), the incubation temperature was 30oC.
2.3.3. Minimum inhibitory concentration testing
Dilution of Ag/PVA nanocomposites was achieved by a series solution technique through changing volume ratios of Ag/PVA nanocomposites to sterile DI water from 1:1, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, to 1:512 in sterile polypropylene tubes. Then the same volume of bacterial solution was added into the dilute Ag/PVA nanocomposite solution. Negative control tubes only contained inoculated broth. The tubes were incubated at 35oC (E. coli, S.aureus and P. aeruginosa) or 30oC (C. albicans) for 18-24 h. The visual turbidity in the tubes was monitored both before and after incubation. Aliquots from tubes (10mL) that appeared to have little or no cell growth were put on LB agar plates to distinguish between bacteriostatic or bactericidal effects. After incubated at 35oC (E. coli, S.aureus and P. aeruginosa) or 30oC (C. albicans) for 16-20 h, the colonies were quantified.
2.3.4. Extended antibacterial testing
Required amounts of Ag/PVA nanocomposites and 4 mL of LB broth containing approximately 5×105 cfu/mL of the bacteria were added orderly to 14 mL of sterile polypropylene tubes (Falcon). And then the tubes were incubated for 18-24 h at 35oC or 30oC. Turbidity was visually monitored after incubation finished. Aliquots of tubes (100 mL) that have little or no cell growth (LB broth clear) were plated on LB plates. The plates were incubated at 35oC (E. coli, S.aureus and P. aeruginosa) or 30oC (C. albicans), and then colonies were quantified. Then the LB was carefully decanted (composites and polymers were insoluble and remained behind), and fresh bacterial suspension in LB (4 mL of 5×105 cfu/mL of the bacteria) was added. The procedure was then repeated for 4 times.
2.4. Characterization
The morphologies and electron diffraction (ED) of the obtained samples were determined by a JEX1200EX transmission electron microscope (TEM)(Japan). Samples for TEM measurement were prepared by placing a drop of colloidal sample on carbon coated copper grid and dried at room temperature. The morphological images of the samples were also determined by a SPI3800N atomic force microscope (Japan) with N4Si3 cantilever. Samples for AFM measurement were prepared by placing a drop of colloidal sample on fresh flaked mica and dried at room temperature. X-ray diffraction analysis was carried out using an Model D/Max 2500 X-ray diffractometer (Rigaka) with Cu Ka radiation. UV-Vis spectra were collected on a Shimadzu UV-3100 spectrophotometer.
3. RESULTS AND DISCUSSION
3.1. The composite synthesis and characterization
The synthetic scheme for preparing Ag/PVA nanocomposites is illustrated in Scheme 1.
Scheme 1. The preparation of Ag/PVA nanocomosite colloids
The golden-yellow solution is stable over a long period of time, and the nanoparticles almost show no tendency to agglomerate, thus indicating effective capping by PVA. On the other hand, the Ag colloid without PVA added deposited in a few days.
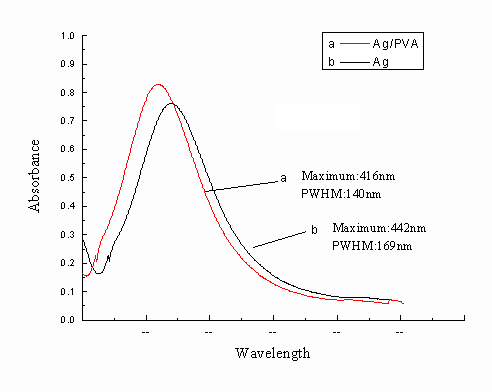
Fig.1 UV-Vis absorption spectra of Ag/PVA and Ag colloids
The UV-Vis absorption spectra of the Ag/PVA colloids are shown in
Fig.1. A maximum absorption located at 416nm for Ag/PVA resulted from the surface plasma
resonance of the electrons in the conduction bands of silver. Under the same experimental
and measurement conditions, the absorption profile of the Ag/PVA nanocomposites is not
very similar to that of Ag nanoparticles without the stabilization of PVA. The absorption
peak of the Ag/PVA colloids was sharper and more symmetric than Ag nanopaticles without
adding PVA, which suggested that the size distribution of the PVA-protected Ag
nanoparticles is uniform and well-dispersed. Compared with that of Ag/PVA nanocomposites, lmax of Ag
nanoparticles shifted to a longer wavelength 442nm. The peak width at half of maxima
(PWHM) is considered to be quite useful parameter in understanding the particle size and
its distribution. The PWHM for Ag particles in the absence of PVA is about 169nm, which is greater than that
for the Ag/PVA nanocomposites(140nm). This indicated a narrower size distribution of the Ag
particles in Ag/PVA.
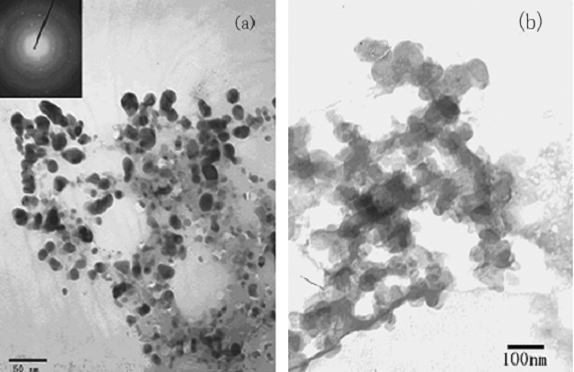
Fig.2 TEM images of Ag/PVA nanocomposite colloids (a) and nano-Ag without PVA(b)
The morphologies of the Ag/PVA nanocomposites and the Ag nanoparticles were investigated by transmission electron microscopy (TEM) (Fig.2). It could be seen from Fig.2a that the PVA-protected Ag particles were spherical and well-dispersed without agglomeration with diameter range of 15-20nm.
The insert in Fig.2a was a typical electron diffraction pattern indicating that silver nanoparticles were crystalline. On the other hand, the nano-Ag particles without protecting of PVA in Fig.2b dispersed irregularly and agglomerated largely and the grain size was over 60nm.The formation of the Ag/PVA nanocomposites was also confirmed by AFM images. Typical AFM images of surface morphology were shown in Fig.3. The particles were spherical and no agglomeration was observed, indicating that PVA molecules efficiently stabilized the nanoparticles to avoid agglomeration. AFM grain analysis indicated the Ag/PVA had an average size of 30nm and a narrow size distribution.
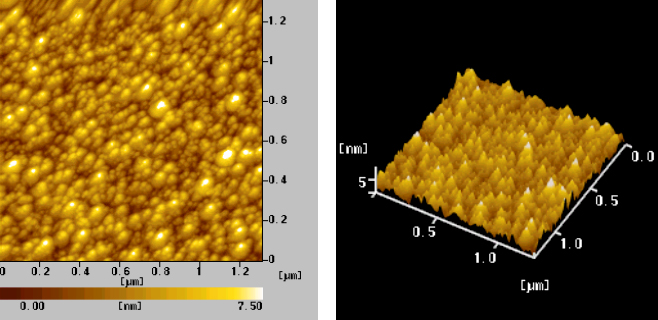
Fig.3 AFM morphological images of Ag/PVA nanocomposite colloids
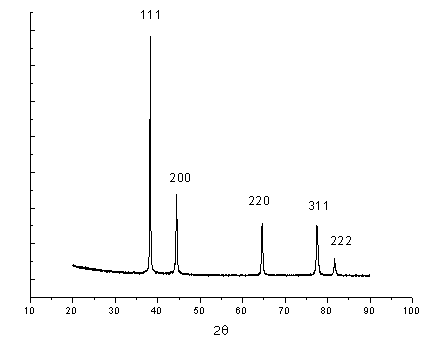
Fig. 4 XRD pattern of Ag/PVA nanocomposites
A typical XRD pattern of the Ag/PVA nanocomposites (Fig.4) showed that five diffraction peaks at 2q
of 38.2°, 44.4°, 64.5°, 77.5° and 81.6° respectively could be indexed as (111), (200), (220), (311) and (222) Bragg's reflections of the face-centered cubic (fcc) structure of silver. The particle size could be calculated from the Ag(111) diffraction line using Scherrer's equation L = kl /b cos q, where L was the mean dimension of the crystallites, b was the full width at half maximum of the diffraction peak, q was the diffraction angle, l was the wave length of Cu K radiation (0.1540nm), and K equaled 0.89. The calculated size of silver nanoparticle protected by PVA was about 25nm.3.2. Antibacterial Effect
The antibacterial properties of the nanocomposites were tested against gram-positive S. aureus and gram-negative E. coli. Fig.5 shows typical antibacterial test results of Ag/PVA nanocomposites and Ag nanoparticles against S. aureus and E. coli determined by a disk method. As shown in Fig. 5, antibacterial ability, measured by the diameter of the growth inhibition zone, depended on the test sample used. PVA impregnated filter paper exhibited no zones of inhibition. Only slight zones of inhibition around the Ag nanoparticles without the stabilization of PVA were observed (Fig. 5b), but Ag/PVA nanocomposites placed on the bacteria-inoculated surface killed all S. aureus bacteria under and around them, distinct zones of inhibition around the Ag/PVA nanocomposites were distinguished (Fig. 5a). The difference of the sizes of zones of inhibition between the Ag nanoparticles and Ag/PVA nanocomposites was caused by the difference of the particles sizes. Further experiments showed that the size of zones of inhibition increased with decreasing size of the Ag nanoparticles. For E. coli similar results were observed(Fig. 5c,d). We attribute this to higher ration of disengaging from the smaller particles due to their higher surface-to-volume ratio. Therefore, it is possible to control the antibacterial activity by varying the size of the Ag particles.
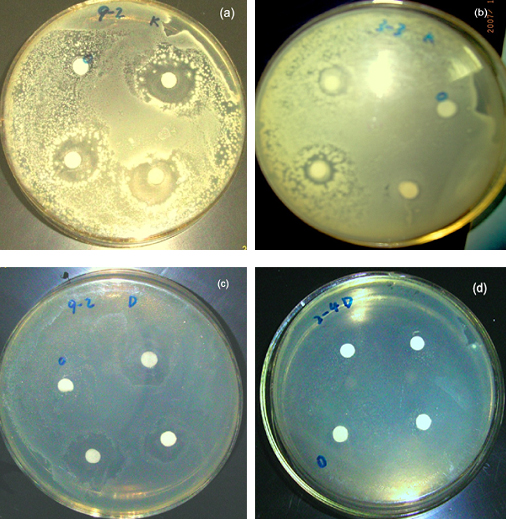
Fig.5 Images of antibacterial activities to S. aureus (a,b)and E. coli of Ag colloids (c,d). (a,c) PVA-protected, (b,d) without PVA
The minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) are the lowest concentrations (mg/mL) at which a compound can respectively inhibit bacterium growth or kill more than 99% of the added bacteria. A lower MIC corresponds to a higher antibacterial effectiveness. The antibacterial activities of Ag/PVA nanocomposites against gram-negative E. coli and gram-positive S. aureus were studied in aqueous LB broth through MIC test. A standard testing protocol for water insoluble antimicrobials was used. Bacterial growth was monitored by visually inspecting turbidity of the LB broth (bacterial growth causes clear LB broth to turn turbid). Lack of turbidity may correspond to either very low bacterial growth (bacteriostatic effect) or complete killing of bacteria (bactericidal effect). To confirm whether the composites were bacteriostatic or bactericidal, 100mL aliquots were taken from the incubated LB broth and put on nutrient agar plates. The plates were then incubated at 35°C for 16-24 h, and then colonies were counted. Bacterial colonies indicated the presence of live bacteria in the aliquots that were plated. If the tested material does not kill but inhibits the growth of bacteria (bacteriostatic), bacteria will grow when taken out from the solution containing the material and plated the fresh LB broth and the colonies will be observed. If the tested material is bactericidal, no bacterial colonies would be observed upon plating. The results are given in Table 1. All the prepared Ag/PVA nanocomposites were bacteriostatic and bactericidal to Gram positive and Gram negative bacteria at an MIC range of 0.3768-1.206mg/mL and an MBC range of 1.206-3.015mg/mL. Clearly, the Ag/PVA nanocomposites exhibited a higher antibacterial activity for both E. coli and S. aureus. These results also supported the dual action mechanism of antibacterial activity viz. the bactericidal effect of Ag+. The results aslo show that the Ag/PVA nanocomposites have a high bactericidal activity against P. aeruginosa. However, the composite nanoparticles can only inhibit growth of the epiphyte C. albicans but not kill them.
Table 1 MIC and MBC of Ag/PVA nanocomposite colloids against different bacteria
|
E.coli |
S. aureus |
P. aeruginosa |
C. albicans |
MIC(mg/ml) |
0.603 |
0.3768 |
0.753 |
1.206 |
MBC(mg/ml) |
1.206 |
3.015 |
3.015 |
- |
Interestingly, the zones of inhibition of the Ag/PVA nanocomposites got larger than that of the Ag nanoparticles without PVA after four days. It indicated that PVA protected silver nanoparticles could prolong the antibacterial activity.
4.CONCLUSION
In summary , the synthesis of Ag/PVA nanocomposites by a simple and easy route was
described in the presence of PVA as a structure director. The prepared composite
nanoparticles had a smaller grain size and more narrow grain distribution. PVA can
efficiently protect the nanoparticles from aggregation. The composites had a very
antibacterial activity towards both gram-positive and gram-negative bacteria.
ACKNOWLEDGEMENTS The authors are grateful to Academy of West-China Resource Applications and Key Lab of Polymer Materials of Gansu province for their financial supports.
REFERENCES
[1] Russel A D, Hugo W B. Prog Med Chem. 1994, 31: 351.
[2] Zachariadis C P, Hadjikakou S K, Hadjiliadis N, et al. Eur J Inorg Chem. 2004, 7: 1420
[3] Hollinger M A. Crit Rev Toxicol.1996, 26: 255
[4] Gosheger G, Hardes J, Ahrens H, et al. Biomaterials. 2004, 25: 5547.
[5] Percival S L, Bowler P G, Russell D. J Hosp Infect. 2005, 60: 1
[6] Brady M J, Lisay C M, Yurkovetskiy A V, et al. Am J Infect Control. 2003, 31: 208
[7] Baker C, Pradhan A, Pakstis L, et al. Nanosci Nanotechnol. 2005, 5: 244.
[8] Aymonier C, Schlotterbeck U, Antonietti L, er al. Chem Commun. 2002: 3018.
[9] Melaiye A, Sun Z, Hindi K, et al. J Am Chem Soc. 2005, 127: 2285.
[10] Sondi I, Salopek-Sondi B, J Colloid Interf Sci. 2004, 275: 177.
[11] Alt V, Bechert T, Steinrucke P, er al. Biomaterials. 2004, 25I: 4383.
[12] Sun R W, Chen R, Chung N P, et al. Chem Comm. 2005: 5059.
[13] Kim TG, Kim Y W. J Mater Res. 2004, 19: 1400.
[14] Pastoriza-Santos I, Liz-Marza'n L M. Nano Lett. 2002, 2: 903.
[15] Li X, Zhang J H, Xu W Q, et al. Langmuir. 2004, 20: 1298.
[16] Muniz-Miranda M. J Raman Spectrosc. 2004, 35: 839.
[17] Mbhele Z H, Salemane M G, van Sittert J M et al. Chem Mater. 2003, 15: 5019.
[18] Zhou Y, Yu S H, Wang C Y, Li X G, et al. Adv Mater.1999, 11 : 850.
[19] Chou K S, Ren C Y. Mater Chem Phys.2000, 64: 241.
[20] Khanna P K, Gokhale R, Subbarao V V V S. J Mater Sci. 2004, 39: 773.
[21] Zhang Z, Zhao B, Hu L. J Solid State Chem. 1996, 121: 105.
[22] He R, Qian X, Yin J, et al. Chem Phys Lett. 2003, 369: 454.
[23] Wu D, Ge X, Huang Y,et al. Mater Lett. 2003,
57: 3549.
[24] Monti O L A, Fourkas JT, Nesbitt D J. J Phys Chem B . 2004, 108: 1604.
[25] Fussell G, Thomas J, Scanlon J, et al. J Biomater Sci Polym Ed. 2005, 16: 489.
[26] Porel S, Singh S, Harsha S S, et al. Chem Mater. 2005, 17: 9.
[27] Heilmann A. Polymer films with embedded metal nanoparticles. New York:
Springer-Verlag, 2002.
[28] Dowling D P, Betts A J, Pope C, et al. Surf Coat Technol. 2003, 163I: 637.
[29] Affinito J, Martin P, Gross M, et al. Thin Solid Films. 1995, 270: 43.
[30] Dowling D P, Donnelly K, McConnell M L, et al. Thin Solid Films. 2001, 398: 602.
[31] Jiang H, Manolache S, Wong A C L, et al. J Appl Polym Sci. 2004, 93: 1411.
[32] Favia P, Vulpio M, Marino R et al. Plasmas Polym. 2001, 5: 1.
[33] Melaiye A, Sun Z, Hindi K,et al. J Am Chem Soc. 2005, 127: 2285.
[34] Khanna P K, Singh N, Charan S,et al. Mater Chem Phys. 2005, 93: 117
薄丽丽 杨武 薛群基 高锦章
(西北师范大学化学化工学院,西部资源应用研究院,兰州 730070)
摘要 使用硝酸银,硼氢化钠,聚乙烯醇分别作为前驱体、还原剂和稳定剂,通过溶液还原法制备了均匀分散的银/聚乙烯醇纳米复合物溶胶,利用UV-Vis光谱,X-射线粉末衍射,透射电子显微镜和原子力显微镜技术对纳米复合物的结构和形貌进行了系统表征。研究表明该复合物对革兰氏阳性菌和革兰氏阴性菌具有强的杀灭活性。
关键词 银纳米复合物溶胶,抗菌性,聚乙烯醇,化学合成