http://www.chemistrymag.org//cji/2008/101005pe.htm |
Jan. 1,
2008 Vol.10 No.1 P.5 Copyright |
Kinetics and mechanism of oxidation of glycyglycine by diperiodatoargentate(III) in aqueous alkaline medium
Shan
Jinhuan, Wang Heye, Song Changying, Wang Fang, Shen Shigang
(College of Chemistry and Environmental Science, Hebei University, Baoding, Hebei 071002,
China)
Abstract The kinetics of oxidation of
glycyglycine by diperiodatoargentate(III) complex (DPA) was studied spectrophotometrically
in aqueous alkaline medium in a temperature range of 293.2K-308.2K. The reaction was found
to be first order with respect to DPA and 1.30-1.35 to glycyglycine. The observed rate
constant (kobs) decreased with the increase of the [IO4-],
decreased with the increase of the [OH-], and then increased with the
increase of the [OH-] after a turning point. The kobs was
independent of the ionic strength and no free radicals were detected. A possible mechanism
involving a two-electron transfer is proposed and the rate equations derived from the
mechanism can explain all experimental results. The activation parameters, as well as the
rate constants of the rate-determining step have been calculated.
Keywords Glycyglycine; Diperiodatoargentate(III); Redox reaction; Kinetics;
Reaction mechanism
1. INTRODUCTION
In recent years, many researchers have focused on the study of peptide,
especially for its chemical modification [1-3]. Glycyglycine is one of the
important peptides, the kinetics and mechanism of oxidation of glycyglycine can provide
some valuable information for the chemical modification of peptide.
Diperiodatoargentate(III) (DPA) is a powerful oxidizing agent in
alkaline medium with the reduction potential of 1.74V[4]. Jayaprakash Rao and
other researchers have studied DPA as an oxidizing agent for the kinetics of oxidation of
some organic substrates, such as amino acids, reducing sugars and amines [5-10]. But the kinetics of oxidation of small peptides by DPA has almost not been reported
previously. In this paper, the kinetics and mechanism of
oxidation of glycyglycine by dihydroxydiperiodatoargentate (III) is presented.
2. EXPERIMENTAL
2. 1 Materials and Reagents
All chemicals used were of A.R. grade and doubly distilled water was used throughout
the work. The stock solution of glycyglycine was prepared by dissolving an appropriate
amount of sample in doubly distilled water. Then glycyglycine
was converted into potassium salt by addition of equivalent KOH solution. The glycyglycine
was used from its stock solution. KNO3 and KOH were used to maintain the ionic
strength and alkalinity of the reaction, respectively. The stock standard solution of IO4-
was prepared by dissolving KIO4 in doubly distilled water and kept for 24h to
attain the equilibrium.
2. 2 Apparatus Kinetic Measurements
The kinetic measurements were performed on a UV-vis spectrophotometer (TU-1901, Beijing
Puxi Inc., China), which had a cell holder kept at constant temperature (± 0.1oC)
by circulating water from a thermostat (BG-chiller E10, Beijing Biotech Inc.,
Beijing). The kinetics measurement was carried out under
pseudo-first-order conditions [Glycyglycine]0>>[Ag(III)]0
at 293.2K-308.2K. The reaction was initiated by mixing DPA with the glycyglycine solution
which also contained KNO3, KOH and KIO4. The progress of the
reaction was monitored spectrophotometrically at 362nm, which is the maximum absorption
wavelength of DPA. It was verified that there was almost no interference from other
species in the reaction mixture at this wavelength.
2.3 Product analysis and free radical detection
A solution with known concentrations of [DPA] and [OH-] was mixed with an
excess of glycyglycine. The completion of the reaction was marked by the complete fading
of DPA color. After completion of the reaction, the product of the oxidation was
identified as the corresponding aldehyde acid by their
characteristic spot test[12]. The addition of acrylonitrile or acrylamide to
the reaction mixture under the protection of nitrogen, neither changed the rate nor
initiated any polymerization, showing no free radicals in the reaction.
3. RESULTS AND DISCUSSION
3. 1 Evaluation of Pseudo-First Order Rate Constants
Under the conditions of [Glycyglycine]0>>[Ag(III)]0, the plots of ln(At-A∞) versus time are linear, indicating the reaction is first order
with respect to [Ag(III)], where At and A∞ are the absorbance at time t and at infinite time respectively. The
pseudo-first-order rate constants kobs were calculated by the method of least
squares (r≥0.999). To calculate kobs
generally 8-10 values of At within three times the half-life were used. The
values of kobs were average values of at least three independent experiments
and reproducibility of kobs is within the experimental error ±5%.
3.2 Rate dependence on [glycyglycine]
The plots of lnkobs versus ln[glycyglycine] are straight linear (r>0.99) at
fixed [DPA], [IO4-], [OH-], ionic strength
(m) and temperature . From
the slope, the observed reaction order (nap) was found to be 1.30-1.35, and
[glycyglycine]2/kobs versus [glycyglycine] are straight linear with
a positive intercept (r > 0.999) (Fig. 1).
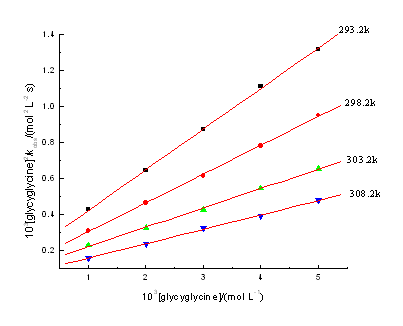
Fig.1 Plots of [glycyglycine]2/kobs
versus [glycyglycine]
[Ag(III)]=4.14×10-5 mol·L-1, [OH-]=0.03mol·L-1,
[IO4-]=0.004mol·L-1, m=0.064mol·L-1.
3.3 Effect of [IO4-]
The concentrations of IO4- were varied from 2.0×10-3 to
4.5×10-3mol·L-1 at constant [DPA], [OH-],
[glycyglycine] and m. It
was observed that the rate constants decreased by increasing [IO4-].
The plots of 1/kobs vs. [IO4-] are straight lines with a
positive intercept (r >0.999) (Fig. 2).
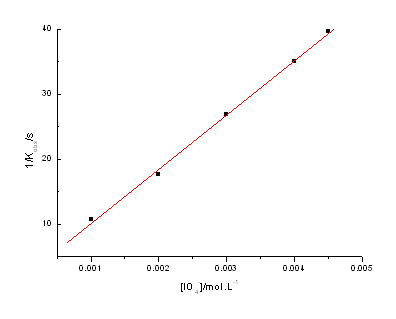
Fig.2 Plots of 1/kobs vs.1/[IO4-]
at 303.2K
[Ag(III)]=4.14×10-5mol·L-1,
[glycyglycine]=0.004mol·L-1, [OH-]=0.03mol·L-1,
m=0.064mol·L-1.
3. 4 Effect of [OH-] and Ionic
Strength (μ)
At constant [DPA], [glycyglycine], [IO4-], m and temperature, the values of
kobs decreased with the increase of [OH-], and then increased with
the increase of [OH-]. The concentration of OH- was approximately
0.02 mol·L-1 at
the turning point, at which the rate was the slowest (Table 1). The addition of KNO3
solution, to adjust the ionic strength of the reaction at constant [DPA], [glycyglycine],
[IO4-], [OH-] and temperature, had no effect on
the rate(Table 2). It showed that there was no salt effect, which was consistent with the
common regulation of the kinetics[13].
Table 1. kobs varying with [OH-] at 303.2K
[OH-] (mol·L-1) |
0.010 |
0.015 |
0.020 |
0.030 |
0.040 |
0.050 |
102kobs (s-1) |
2.862 |
2.771 |
2.712 |
2.864 |
3.140 |
3.357 |
[Ag(III)]=4.14×10-5mol·L-1, [glycyglycine] = 0.004mol·L-1, [IO4-]=0.004mol·L-1, μ=0.064mol·L-1.
Table 2. kobs varying with ionic strength at 303.2K
m(mol·L-1) |
0.050 |
0.064 |
0.100 |
0.150 |
0.200 |
102kobs (s-1) |
2.861 |
2.898 |
2.925 |
2.889 |
2.920 |
[Ag(III)]=4.14×10-5mol·L-1, [glycyglycine]=0.004mol·L-1, [OH-]=0.03mol·L-1, [IO4-]=0.004mol·L-1
4. REACTION MECHANISM
In periodate aqueous solution equilibria (1)-(3) were observed and the corresponding
equilibrium constants at 298.2K were determined by Aveston [14]
2IO4- + 2 OH-![]() H2I2O104-
log b1=15.05
(1)
H2I2O104-
log b1=15.05
(1)
IO4-+OH-+H2O![]() H3IO62-
log b2= 6.21 (2)
H3IO62-
log b2= 6.21 (2)
IO4- + 2 OH-![]() H2IO63-
log b3= 8.67
(3)
H2IO63-
log b3= 8.67
(3)
The distribution of all species of periodate in aqueous alkaline
solution can be calculated from equilibria (1)-(3). In the [OH-] range used in
this work the amount of dimer and IO4- species of periodate is
neglected. The main species of periodate are H2IO63-
and H3IO62-, consistent with the result calculated
from Crouthamel's data[15] by Murthy. Eqs. (4) and (5) can be obtained from(3)
and(2):
![]() (4)
(4)
![]() (5)
(5)
Here [IO4-]ex represents the
concentration of original overall periodate ion and is approximately equal to the sum of [
H2IO63- ] and [H3IO62-].
Based on such distribution, the formula of Ag(III) periodate complex may be represented by
either [Ag( OH)2( H3IO6)2]3-
or the less protonated [Ag( OH)2 (H2IO6)2]5-.
We preferred to use the latter to represent DPA because it is closer to that suggested by Mukherjee[16].
Based on the above discussion, two simultaneous reaction mechanisms
were proposed:
Mechanism I—in weaker alkaline medium:
[Ag(OH)2(H2IO6)2]5- +OH- ![]() [Ag(OH)2(HIO6)]3- +H2IO63-+H2O
(6)
[Ag(OH)2(HIO6)]3- +H2IO63-+H2O
(6)
A
B
-OOCCH2NHCOCH2NH2+H2O ![]() -OOCCH2NHCOCH2NH3++OH- (7)
-OOCCH2NHCOCH2NH3++OH- (7)
R-
RH
[Ag(OH)2(HIO6)]3-+RH
![]() [Ag(OH)2(HIO6)(RH)]3-
(8)
[Ag(OH)2(HIO6)(RH)]3-
(8)
B
adduct
[Ag(OH)2(HIO6)(RH)]3-+R-![]() [Ag(OH)2(HIO6)(RH)]3-R-
(9)
[Ag(OH)2(HIO6)(RH)]3-R-
(9)
[Ag(OH)2(HIO6)(RH)]3-R- ![]() product
(10)
product
(10)
The total concentration of Ag(III) at time t can be written as
[Ag(III)]t=[A]e+[B]e
+[adduct]e. Since reaction (9) is
the rate-determining step, the rate of disappear of [Ag(III)]t is represented
as:
-d[Ag(III)]t/dt=k·[adduct]e·[R-]
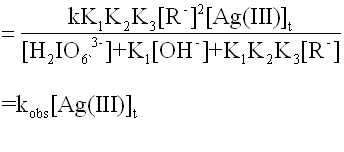 (11)
(11)
kobs=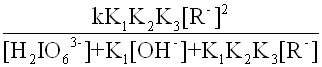 (12)
(12)
Equation(13) can be obtained from equation(4) and (12)
kobs= 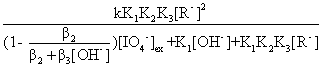 (13)
(13)
![]() (14)
(14)
Mechanism II—in stronger alkaline medium:
[Ag(OH)2(H2IO6)2]5- +OH- ![]() [Ag(OH)2(HIO6)]3-
+H2IO63-+H2O (15)
[Ag(OH)2(HIO6)]3-
+H2IO63-+H2O (15)
A
B
[Ag(OH)2(HIO6)]3- +R-![]() [Ag(OH)2(HIO6)(R-)]4-
(16)
[Ag(OH)2(HIO6)(R-)]4-
(16)
B
adduct
[Ag(OH)2(HIO6)(R-)]4-+R- ![]() [Ag(OH)2(HIO6)(R-)]4-R-
(17)
[Ag(OH)2(HIO6)(R-)]4-R-
(17)
[Ag(OH)2(HIO6)(R-)]4-R-![]() product (18)
product (18)
The total concentration of Ag(III) at time t can be written as
[Ag(III)]t=[A]e+[B]e
+[adduct]e. The reaction (17) is
the rate-determining step, the rate of disappear of [Ag(III)]t is represented
as:
-d[Ag(III)]t/dt=k·[adduct]e·[R-]
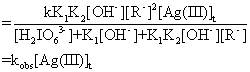 (19)
(19)
kobs =![]() (20)
(20)
Equation(21) and (22) can be obtained from equation (20) and (4)
![]() (21)
(21)
![]() (22)
(22)
![]() (23)
(23)
Equation (13) and (22) can explain the values of kobs
decreased rapidly with the increase of [OH-] up to the lowest point. After the
point, it increased gradually with the continuous increase of [OH-]. Equation
(21) shows that the plots of 1/kobs versus [IO4-]ex
should be linear(evidenced by Fig.2). Equation (20) shows that the order in glycyglycine
should be 1< nap <2 and Equation (23) shows that [glycyglycine]2/kobs
versus [glycyglycine] should be linear (confirmed by Fig.1). Meanwhile, the
plots of [glycyglycine]2/kobs versus
[glycyglycine] were linear at different temperatures. From Equation (23), it can be seen
that the slope of the linear plot of [glycyglycine]2/kobs versus
[glycyglycine] is 1/k. From the slopes in Fig.1, the values of k at different temperature
were evaluated. The activation parameters were evaluated by the method given earlier[17].
Rate constants and activation parameters are listed in Table 3. The negative DS≠supports the view that the rate-limiting step consists in the
formation of an intermediate complex and does not involve the breaking of a bond.
T /K |
k/mol-1·L·s-1 |
activation parameters |
293.2 |
1.49 |
Ea=52.547 kJ·mol-1 |
298.2 |
1.85 |
|
303.2 |
2.26 |
|
308.2 |
2.52 |
The regression equation of glycyglycine is shown: lnk=23.06-6320.31/T, (r=0.997)
REFERENCES
[1] Feeny RE. Int J Pept Proteins, 1987, 29: 145-161.
[2] Criado S et al. Journal of Photochemistry and Photobiology B: Biology, 2001, 65: 74–84.
[3] Lazzaro F et al. Tetrahedron Letters, 2004, 45 : 9237–9240.
[4] Sethuram B. Some Aspects of Electron Transfer Reactions
Involving Organic Molecules. New Delhi: Allied Publishers (P) Ltd, 2003 :151.
[5] Rao P J, Sethuram B , Rao T N. React. Kinet. Catal. 1985, 29: 289-296.
[6] Krishna K V, Rao P J P. Transition Met Chem, 1995, 20 (1): 344-346.
[7] Kumar A, Vaishali, Ramamurthy P. Int J Chem Kinet, 2000, 32: 286-293.
[8] Sarala G, Rao P J P, Rao T
N. Indian J.Chem, 1987, 26A: 475-480.
[9] Shan J H, Li S M, Huo S Y. Transition Met Chem, 2005, 30 (6): 651-654.
[10] Kumar A et al. Polyhedron, 1999, 18 : 773.
[11] Cohen G L, Atkinson G. Inorg.Chem, 1964, 3 : 1741.
[12] Feigl F. Spot Tests in Organic Analysis. New York : Elsevier Publishing Co, 1956:
208.
[13] Jin J J. Kinetics Principie of Chemical Reaction in Liquid Phase. Shanghai: Science
Technique Press, 1984: 186-218.
[14] Aveston J. J. Chem. Soc (A), 1969, 273.
[15] (a) Crouthamel C E, Meek H V, Martin D S et al. J. Am. Chem.Soc, 1949, 71: 3031.
(b) Crouthamel C E, Meek H V, Martin D S et al. J.
Am. Chem.Soc, 1951, 73: 82.
[16] Mukherjee H G, Mandal B, De S. Indian J. Chem, 1984, 23A: 426.
[17] Shan J H, Liu T Y. Acta Chimica Sinica, 1994, 52: 1140.
单金缓 王合叶 宋常英 王芳 申世刚
(河北大学化学与环境科学学院,保定 071002)
摘要 采用分光光度法在293.2K-308.2K温度范围研究了碱性介质中二羟基二(过碘酸根)合银(III)配离子(DPA)对双甘肽的氧化还原反应动力学及机理。结果表明:反应对DPA是准一级反应,对双甘肽的反应级数为1.30-1.35;在保持准一级条件下([glycyglycine]0>>[DPA]0),反应表观速率常数随着[OH-]的增加先减小后增加,随着[IO4-]的增加而减小;无明显的盐效应且没有自由基检测出来。根据实验结果提出反应机理,由此得出反应速率方程式,并能解释所有的实验现象。同时根据温度对反应速率的影响,进一步求得速控步骤速率常数k,估算出反应的活化能并提出DPA氧化双甘肽的机理。
关键词 二羟基二(过碘酸根)合银(III) 双甘肽 氧化还原反应 动力学 反应机理