http://www.chemistrymag.org//cji/2008/102006pe.htm |
Feb. 1,
2008 Vol.10 No.2 P.6 Copyright |
(College of Chemistry and Environmental Science, Hebei University, Baoding 071002)
Abstract A new assay of phosphorus is
proposed by using of resonance light scattering (RLS) with high selectivity and
sensitivity. In 0.25 mol/L H2SO4 solution, the interaction between
molybdophosphoric heteropoly acid (HPAs) and methyl green results in a great enhancement
of RLS signals in the wavelength range from 200 to 700 nm characterized by the peak around
367 nm. The enhanced RLS intensity at 367 nm is proportional to the concentration of
phosphorus in the range of 0.1-200 ng/mL and the detection limit (3s) is 0.04 ng/mL. Therefore a sensitive
method for phosphorus determination is established.
Keywords resonance light scattering (RLS), methyl green, molybdophosphoric
heteropoly acid (HPAs)
1. INTRODUCTION
As one of the key elements in the biogeochemical cycling for the growth of plants and
animals, phosphorus is an essential mineral required by every life. The majority of the
phosphorus in the body is found as phosphate (PO43-). However, if an
excess of phosphate enters in the aquatic system, algae and aquatic plants will grow
wildly, choke up the aquatic system and use up large amounts of oxygen. This situation is
known as eutrophication or overfertilization of receiving waters, which results in the
death and decay of vegetation and aquatic life. Therefore, a highly sensitive and simple
analytical method is very necessary to obtain a better understanding of the biogeochemical
cycling for life science and to protect the environments.
Spectrophotometric method based on the formation of the blue (reduced)
form of molybdophosphoric heteropoly acid (HPAs) is the most wildly used for phosphate
determination [1]. The limit of detection (LOD) for the spectrophotometric
method is typically 10 ng/mL P, which exhibites a low sensitivity. Various methods for
phosphate determination based on enzymatic coupled reactions have also been developed [2-4],
in which phosphate is utilized in the coupled reactions yielding products that are
detected by spectrophotometry or spectrofluorimetry. However, these enzymatic methods
generally need complex procedures and expensive reagents, and such are time-consuming and
high cost. High sensitivity of phosphate determinations has been achieved by
chemiluminescence (CL) detection based on oxidation of 3-amino-phthalhydrazide (luminol)
with vanadomolybdophosphoric HPAs, for which the LOD is 4.3 ng/mL P [5]. With
the sorption preconcentration [6] or in-line iminodiacetate chelating column [7],
as low as 0.02 or 0.03 ng/mL P can be detectable, respectively.
Recently, resonance light scattering has become a new interesting
technique for determination of micro amounts of biomolecules [8], such as
nucleic acids [9], proteins [10], and drug [11]. The RLS
methods are very attractive because high sensitivity can be obtained with a common
spectrofluorimeter by using inexpensive reagents. In this paper, it is found that the
associated complex of molybdophosphoric HPAs and methyl green can be formed through strong
electrostate interaction, which results in a great enhancement of RLS signals. The
enhanced RLS intensities at 367 nm are proportional to the concentrations of phosphorus in
the range of 0.1-200 ng/mL and LOD of 0.04 ng/mL can be achieved without preconcentration
by the proposed RLS assay, which is comparable to that of the CL method by using
preconcentration.
2. EXPERIMENTAL
2.1 Apparatus
The RLS intensity and spectra were measured and recorded by a Hitachi F-4500 Fluorescence Spectrophotometer (Tokyo, Japan) equipped with a 1 cm quartz cell. A QL-901 vortex mixer (Jiangsu Clinical Instruments Plant, Haimen, China) was employed to blend the solution.
2.2 Reagents
A 0.04 mol/L stock solution of (NH4)2MoO4 was prepared by dissolving 2.4717 g (NH4)2MoO4 (Tianjin chemical reagents four plant) in 100 mL water. KH2PO4 solution (containing 20 mg/mL Phosphorus) was prepared by dissolving 0.0087 g KH2PO4 (Tianjin chemical reagents four plant) in 100 mL water. Working solutions were prepared by diluting the stock solution. Methyl green solution (4×10-4 mol/L) was prepared by dissolving 0.0207 g methyl green (Tianjin chemical reagents four plant) in 100 mL water. All reagents were of analytical grade, and doubly distilled water was used throughout.
2.3 Procedures
In a 10 mL volumetric flask, 0.25 mL of (NH4)2MoO4 solution (0.04 mol/L), a certain volume of KH2PO4 working solution, 1.0 mL of H2SO4 solution (0.25 mol/L), 0.5 mL of methyl green solution (4×10-4 mol/L) were added in turn, the mixture was diluted to 10.0 mL with water and stirred thoroughly. After incubation at room temperature for 20 min, the RLS spectra were obtained by synchronously scanning with the same excitation and emission wavelength by F-4500 Spectrophotometer in the wavelength range of 200~700 nm. The RLS intensity was measured at the wavelength 367 nm and the slit width and PMT voltage of the measurements were 5 nm and 400 V, respectively.
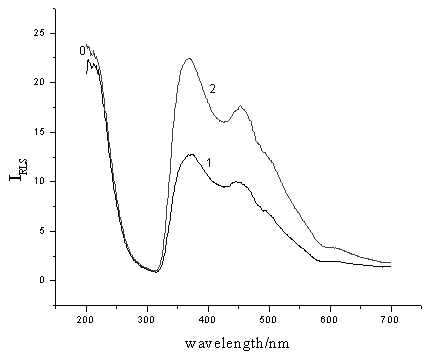
Fig.1 Resonance light-scattering spectra of molybdophosphoric HPAs-methyl green/system. 1: (NH4)2MoO4, 1 mmol/L; H2SO4, 0.25 mol/L; methyl green, 2×10-5 mol/L. 2: (NH4)2MoO4, 1 mmol/L; H2SO4, 0.25 mol/L; methyl green, 2×10-5 mol/L; KH2PO4, containing 1 ng/mL phosphorus. 3. RESULTS AND DISCUSSION
3.1 Spectral characteristics of RLS
Fig. 1 shows the RLS spectra of the mixture of (NH4)2MoO4 and methyl green in the absence and in the presence of phosphate. The mixture of (NH4)2MoO4 and methyl green exhibites two weak RLS peaks at 367 nm and 454 nm, respectively. In the presence of phosphate, the molybdophosphoric HPAs can be formed. As a large molecule with negetive charges, the HPAs can combine with the methyl green, a cationic dye, through strongly electrostate interaction to form an associated complex, which results in the strong enhancement of RLS. Because the RLS intensity at 367 nm is much stronger than that at 454 nm, the former was chosen as the optimum wavelength for RLS measurements of phosphorus in order to obtain high sensitivity.
3.2 Optimization of the reagent condition
3.2.1 Effect of concentration of H2SO4 solution
The concentration of H2SO4 has great effect on the formation of the molybdophsphoric HPAs. So the influence of H2SO4 concentration on the RLS intensity was investigated in the range of 0.05-0.35 mol/L. As can be seen from Fig. 2 that the RLS intensities are stable when the concentration of H2SO4 was in the range of 0.05-0.35 mol/L and the enhanced RLS intensity reaches its maximum at 0.25 mol/L. So 0.25 mol/L H2SO4 was elected for subsequent work.
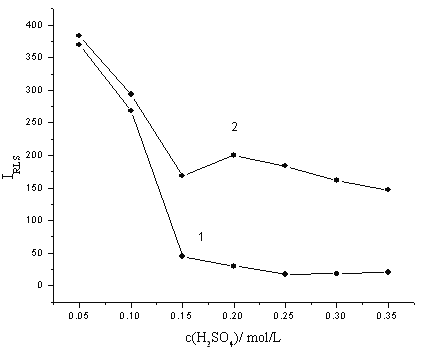
Fig. 2 The effect of the concentration of H2SO4. 1: (NH4)2MoO4, 1 mmol/L; methyl green, 2×10-5 mol/L. 2: (NH4)2MoO4, 1 mmol/L; methyl green, 2×10-5 mol/L; KH2PO4, containing 20 ng/mL phosphorus
3.2.2 Effect of (NH4)2MoO4 concentration
The effect of (NH4)2MoO4 concentration on the RLS intensity was also studied. As shown in Fig. 3, with increasing the (NH4)2MoO4 concentration, the enhanced RLS intensity was greatly increased when (NH4)2MoO4 concentration was less than 1 mmol/L, and decreased when the concentration was greater than 1 mmol/L. On the other hand, the blank RLS intensity was increased when the concentration was greater than 2 mmol/L. So 1 mmol/L (NH4)2MoO4 was selected as the optimum in this assay.
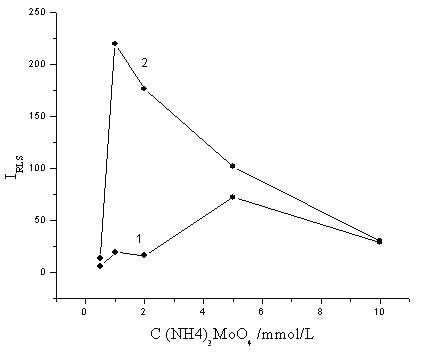
Fig. 3 The effect of the (NH4)2MoO4 concentration. 1: methyl green, 2×10-5 mol/L. H2SO4, 0.25 mol/L; 2: methyl green, 2×10-5 mol/L; H2SO4, 0.25 mol/L; KH2PO4, containing 20 ng/mL phosphorus.
3.2.3 Effect of concentration of methyl green
Fig. 4 shows the effect of methyl green concentration on the RLS intensity in the concentration range from 2.5×10-6 to 5.0×10-5 mol/L. As show in Fig. 4, the RLS intensity greatly increased with increasing the concentration of methyl green when the concentration was less than 5.0×10-6 mol/L and then became stable in the range of 1.0×10-5~5.0×10-5 mol/L. Therefore, 2.0×10-5 mol/L was selected as the optimum concentration of the methyl green.
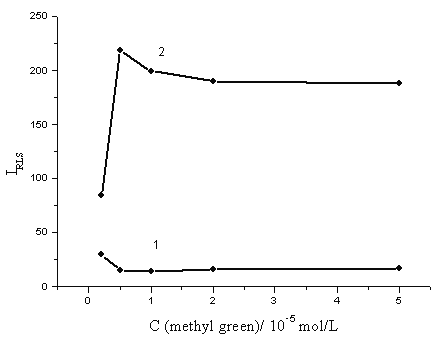
Fig. 4 The effect of the methyl green concentration. 1: (NH4)2MoO4, 1 mmol/L; H2SO4, 0.25 mol/L; 2: (NH4)2MoO4, 1 mmol/L; H2SO4, 0.25 mol/L; KH2PO4, containing 20 ng/mL phosphorus
3.2.4 Effect of ionic strength and incubation time
The effect of ionic strength on the RLS intensity was explored by the addition of NaCl in the concentration range of 0.01-0.5 mol/L. It can be seen from Fig. 5 that the ionic strength has little effect on the RLS intensities, which indicates that the electrostatic interaction between the mdybdophosphoric HPAs and methyl green was strong, and the formed associated complex was very stable.
The influence of the incubation time of the molybdophosphoric HPAs-methyl green solution on the RLS intensity was also investigated. It can be seen from Fig. 6 that the RLS intensity of the formed associated complex could be reached its maximum immediately when the solutions were mixed, and remained constant at least for 120 min. The results indicated that the stability of the RLS signal is practical for the determination of phosphorus.
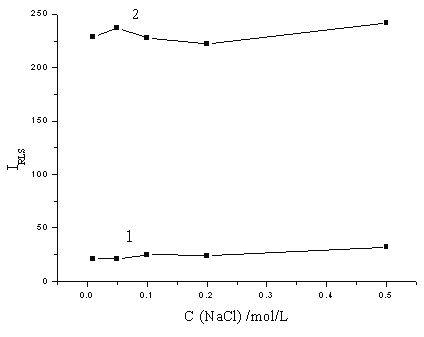
Fig. 5 The effect of ionic strength. 1: (NH4)2MoO4, 1 mmol/L; H2SO4, 0.25 mol/L; methyl green, 2×10-5 mol/L. 2: (NH4)2MoO4, 1 mmol/L; H2SO4, 0.25 mol/L; methyl green, 2×10-5 mol/L; KH2PO4, containing 20 ng/mL phosphorus
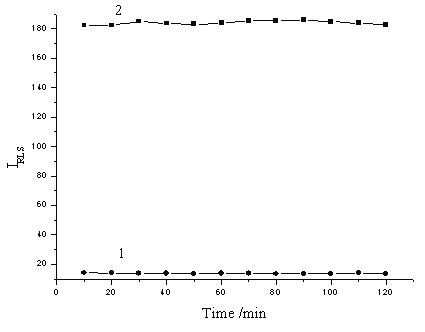
Fig. 6 The effect of incubation time. 1: (NH4)2MoO4, 1 mmol/L; H2SO4, 0.25 mol/L; methyl green, 2×10-5 mol/L. 2: (NH4)2MoO4, 1 mmol/L; H2SO4, 0.25 mol/L; methyl green, 2×10-5 mol/L; KH2PO4, containing 20 ng/mL phosphorus
3.3 Calibration curves
According to general procedure, the calibration curves for phosphorus determination were constructed under the optimum conditions. There is a linear relationship between the enhanced RLS intensity (DIRLS) and phosphorus concentration (C) in the range of 0.1~200 ng/mL. The linear regression equation of this assay is DIRLS = 38.08 + 8.183 C, the correlation coefficient is 0.996 and the detection limit (3s) is 0.04 ng/mL.
4.
CONCLUSIONWe have demonstrated that the molybdophosphoric HPAs and methyl green can form the stable associated complex, which produces the strong RLS signal. The enhanced RLS intensities are proportional to the concentration of phosphorus in a wide range. Based on this, a highly sensitive and very convenient RLS method for phosphorus determination can be developed. The sensitivity increases more than two orders of magnitude greater than that of molybdophosphoric blue based spectrophotometric method and is comparable to that of chemiluminescent method with preconcentration, which is one of the most sensitive approaches available to the phosphorus determination. Therefore, the proposed RLS method may open up a new possibility for phosphorus determination and has great potential in biogeochemical and environmental fields.
Acknowledgement The project was supported by the National Science Foundation of China (NSFC, No. 20675023) and the National Science Foundation of Hebei Province (No. B2006000967).
REFERENCES
[1] Analytical chemistry experiment. Edited by Wuhai University. The fourth section.
Beijing, Higher Education Press, 2001.
[2] Vazquez M J, Rodriguez B, Zapatero C et al. Anal Biochem, 2003, 320: 292.
[3] Conrath N, Grundig B, Huwel S et al. Anal Chim Acta, 1995, 309: 47.
[4] Hill M, Arrio B. Anal Biochem, 1997, 254: 249.
[5] Lukovskaya N M, Bilochenko V A. Ukr Khim Zh, 1977, 43: 756.
[6] Zui O V, Birks J M. Anal Chem, 2000, 72: 1699.
[7] Yaqoob M, Nabi A, Worsfold P J. Anal Chim Acta, 2004, 510: 213.
[8] Huang C Z, Li Y F. Anal Chim Acta, 2003, 50: 105.
[9] Huang C Z, Li K A, Tong S Y. Anal Chem, 1997, 69: 514.
[10] Li Z P, Liu C H, Su Y Q et al. Chem J on Internet, 2005, 7 (12): 83.
[11] Feng P, Shu W Q, Huang C Z et al. Anal Chem, 2001, 73: 4307.
以甲基绿为探针用共振光散射测定磷
(保定河北大学化学与环境科学学院,071002)
摘要 在 0.25 mol/L的硫酸介质中,磷钼杂多酸与甲基绿相互作用导致共振光散射信号在 200-700 nm之间有很大的增强,并且在 367 nm处散射值达到最大。测定磷的标准曲线的线性范围是 0.1-200 ng/mL,检出限是 0.04 ng/mL。基于此现象,研究建立了一种高灵敏的检测磷的方法。
关键词 共振光散射,甲基绿,磷钼杂多酸