http://www.chemistrymag.org//cji/2008/102007ne.htm |
Feb. 1,
2008 Vol.10 No.2 P.7 Copyright |
Influence of heating time on the color and acrylamide formation
Shi Zhihong, Zhao Xi, Zhang Hongyi, Zhou
Jianke, Zhang Li
(College of Chemistry and Environmental Science, Hebei University; Key Laboratory of
Analytical Science and Technology of Hebei Province, Baoding, 071002, China)
Abstract The effect of heating time on
the formation of color and acrylamide was studied. Radix Asparagi, a kind of traditional
Chinese herb, which contains free amino acid asparagine and reducing sugars in relatively
high amounts,was selected as a model. The content of
acrylamide formed during the heating process was determined by GC-ECD and color changes
were detected by a Model 721 spectrophotometer. Experimental results showed that the
formation of acrylamide at 190ºC increased rapidly at the onset of heating, reaching an apparent
maximum value at 15 min and decreasing rapidly afterwards. The same trend was observed in
color changes.
Keywords Acrylamide, heating time, color, Radix Asparagi, GC-ECD
1. INTRODUCTION
In April 2002, detection of high concentrations of acrylamide in common heated
starch-rich foodstuffs by the Swedish National Food Administration attained considerable
public concern, since acrylamide was found to be carcinogenic in rodents and was
classified as a probable human carcinogen [1,2]. Researches have shown that
acrylamide formation is closely linked to the Maillard reaction, which is the
non-enzymatic browning reaction. The free amino acid asparagine and reducing sugars are
considered as the main precursors of acrylamide [3,4]. Radix Asparagi, Chinese
name Tiandong, is a kind of traditional Chinese herb which contains these compounds in
relatively high amounts. So, Tiandong was chosen as a model plant to study on the
relationship between heating time and the formation of acrylamide. The content of
acrylamide was determined by GC-ECD after extraction and derivatization.
Colored products are also formed in foods during heating as a result of
Maillard reaction. Through our experiment, we found that the aqueous extract of the
heating processed herb had different colors at different heating time, so we used
spectrophotometer to detect the aqueous extract to demonstrate the difference between
samples heated for different period of time.
This paper presents the changes in colour and acrylamide levels with
heating time in traditional Chinese medicine Radix Asparagi during heating process.
2. EXPERIMENTAL
2.1 Chemicals and materials
Acrylamide (>99.9%) was purchased from Amresco (Solon, Ohio, USA). Stock solution
of acrylamide (0.1mg/ml) was prepared by dissolving the compound in distilled water. All
other reagents used were of analytical grade. Traditional Chinese herb Radix Asparagi was
purchased from Yixiaotang drug store (Baoding, China).
2.2 Instrumentation
GC analyses were performed on an SP 3400 GC chromatograph (Varian, USA) which
consisted of an electron capture detector (ECD), a 1075 split/splitless injector and an
N-2000 dual channel chromatographic data station. Absorbance analyses were performed on a
721 spectrophotometer (Shanghai Optical Instrument Factory, China).
2.3 Heating process for Radix Asparagi
The Raw material of traditional Chinese herb was cut into slices by stainless steel knife.
Then the pieces were put in a culture dish to form a thin layer. Parallel samples were
baked in a thermostatic-electric oven at 190ºC for 5, 10, 15, 20, 25 min, respectively.
2.4 Sample extraction and derivatization
The baked Chinese herb was pulverized into powder and
homogenized. 1.0 g of the powder was accurately weighed and put in a centrifuge tube, 10ml
of 2 mol/L NaCl solution was added. The centrifuge tube was votexed for 10min and then it
was centrifuged at 4000 rpm for 20 min. 5 ml of the supernatant was put in a 10 ml brown
volumetric flask, 0.6 ml of 10 % H2SO4 was added, then 2 mol/L NaCl
was added to the mark. After being kept at 4ºC in a fridge for 15 min, the centrifuge tube was taken out and 1.0
ml of 0.1mol/L KBrO3 and 1.5 g KBr was added as derivative reagents [5].
The derivatization reaction was completed at 4ºC over night. 0.7 ml of 0.1mol/L Na2S2O3
was added to remove excess bromine. 4ml of the reaction solution was extracted twice
with 4ml of ethyl acetate. The organic phase was dried with anhydrous Na2SO4.
1 m l of the final sample solution was injected for GC-ECD analysis.
2.5 GC analysis of acrylamide
Separation was performed on an FFAP capillary column (50m × 0.22mm × 0.2mm,S.G.
E, Australia) using nitrogen as the carrier gas at a flow rate of 1.25 ml/min, applying
the following temperature program: 120ºC (hold time 0.5 min), then rises at 10ºC·min-1
to 180ºC. The sample was injected by using splitless injection mode, valve
time was 0.5min, injection volume was 1m L. The injector temperature was held at 220ºC and the ECD
detector temperature was held at 300ºC. Peak area was used for
quantification.
2.6 Spectrophotometric determination of the color of the sample
0.25g of the powder was accurately weighed and transfered to
a centrifuge tube, and then it was extracted with 10 ml water for 10 min on a vortex
oscillator. The homogenate was centrifuged at 5000 rpm for 10 min, and the supernatant was
separated and detected at 660nm by using a 721 spectrophotometer.
3. RESULTS AND DISCUSSION
3.1 The GC analysis of acrylamide
Typical chromatograms for the derived products of acrylamide standard and the sample are
shown in Fig. 1. From Fig. 1, it can be seen that the retention time of the derivative of
acrylamide is 16.965 min. The peak of the derivative of acrylamide was not interfered by
the co-existed components in the sample.
To prepare the calibration curve, 0.015, 0.030, 0.075, 0.150 and 0.750 mg/ml of acrylamide standard solution
was derivatized for GC-ECD analysis according to the procedures described in 2.5 and 2.6.
The regression equation was Y= 134947.4 X + 29.4799, correlation coefficient R = 0.99993,
LOD was calculated to be 0.5 mg/kg by signal to noise ratio of 3.
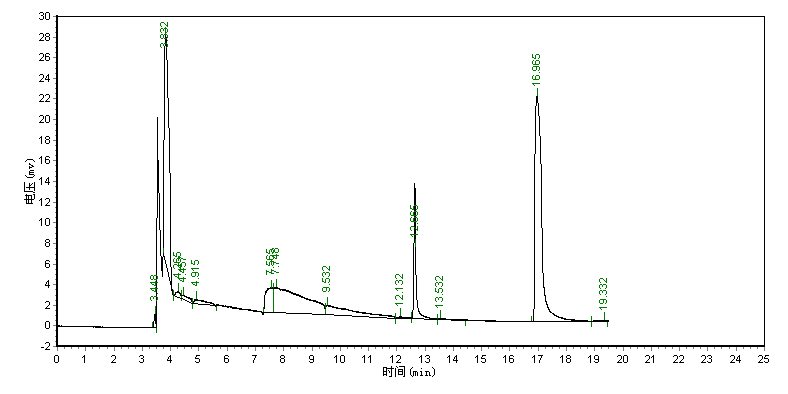
(A)
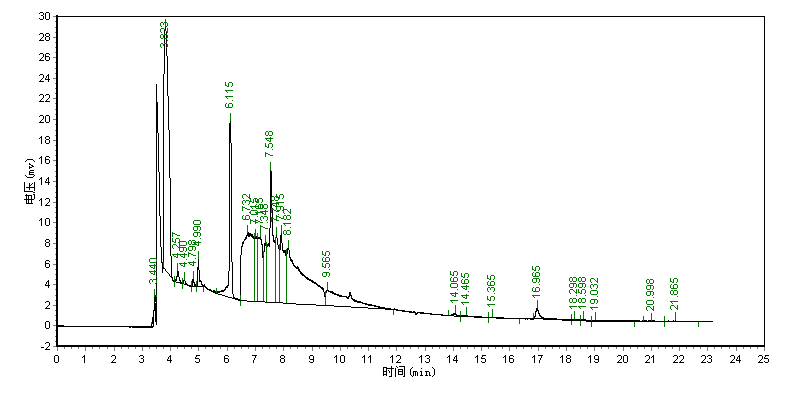
(B)
Fig.1 Chromatograms for derivatization results of acrylamide standard and the sample.
(A) Chromatogram of the derivative of acrylamide standard, (B) Chromatogram of the
derivative of the sample, the retention time of the derivative of acrylamide is 16.965
min.
3.2 The effect of heating time on the
formation of acrylamide
Fig. 2 shows the relationship between acrylamide formation and the heating time for the
herb material baked at 190ºC. From Fig 2, it can be seen that when the material was heated for
5 min, the acrylamide formed was only 853.4 m g/kg, but with the increase of the heating
time, the acrylamide content increased significantly. When the heating time was prolonged
to 15 min, the content of acrylamide in the sample reached 10709.2 m g/kg, which increased
for 12.5 times. While when the heating time was prolonged to 20 min, the acrylamide
content decreased rapidly. After 20 min, the acrylamide content decreased gradually.
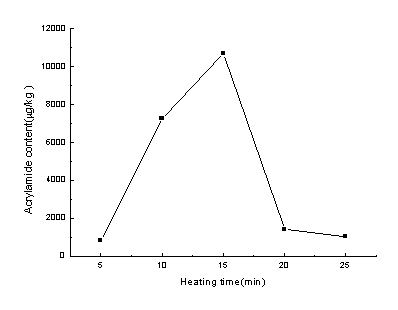
Fig.2 The effect of heating time on the formation of
acrylamide
3.3 The effect of heating time on the
color formation
Acrylamide formation was found to occur during the browning process by Maillard reaction
of reducing sugars with asparagine at temperatures above 120ºC. Colored
products are also formed in the sample during heating process as a result of Maillard
reaction. The heating time also affects the color formation as it does on the formation of
acrylamide. From our experimental results, we found that samples baked at 190ºC for different
period of time presented different depth of colors. To quantitatively demonstrate the
difference of the color of the samples, we extracted the samples with water and determined
the absorbance of the extraction solution at 660 nm by using a 721 spectrophotometer. The
results are shown in Fig.3. While the absorbance of the solution increased rapidly at the
onset of heating, reaching an apparent maximum value at 15 min and decreasing rapidly
afterwards.
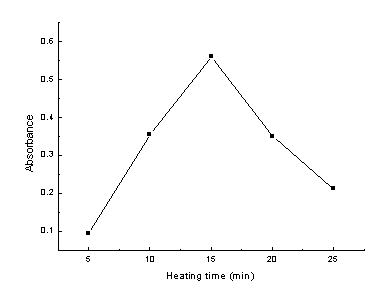
Fig. 3 The effect of heating time on the color
formation
4. CONCLUSIONS
The effect of heating time on the formation of color and acrylamide was investigated
in this paper. It was interesting to see that changes in acrylamide levels and the
absorbance of the aqueous extracts of the sample followed almost the same trend during
heating at 190ºC for different period of time, and an initial increase to a
maximum followed by a subsequent decrease was observed in both cases.
ACKNOWLEDGMENTS Financial support from the National Natural Science Foundation of China (20575016) and the Natural Science Foundation of Hebei Province China (B2006000953) are gratefully acknowledged.
REFERENCES
[1] Friedman M. J. Agric. Food Chem., 2003, 51: 4504-4526.
[2] Senyuva H. Z., GokmenV. Food Addit. Contam., 2005, 22(3): 214-220.
[3] Mottram D. S., Wedzicha B. L., Dodson A. T. Nature, 2002, 419:448-449.
[4] Stadler R. H., Blank I., Varga N. et al. Nature, 2002, 419: 449.
[5] Zhang Y., Dong Y., Ren Y. P. et al. J. Chromatogr.A, 2006, 1116:209-216.
加热时间对丙烯酰胺形成和颜色变化的影响研究
石志红, 赵喜, 张红医, 周建科, 张立
(河北大学化学与环境科学学院, 河北省分析科学技术重点实验室
河北 保定 071002)
摘要 本文以富含天门冬酰胺和还原性糖的药用植物天冬作为研究模型,考察了加热时间对丙烯酰胺的形成和颜色变化的影响。样品中形成的丙烯酰胺经提取、衍生后采用GC-ECD法测定,颜色变化通过测定水提液的吸光度值来表征。研究结果表明:在190ºC,随着加热时间的延长,丙烯酰胺的含量逐渐升高,15
min达最大值,随后丙烯酰胺的含量急遽降低,颜色变化呈现相似趋势。
关键词 丙烯酰胺,加热时间,颜色,天冬,GC-ECD