http://www.chemistrymag.org/cji/2008/102010ne.htm |
Feb. 1,
2008 Vol.10 No.2 P.10 Copyright |
Synthesis of hantzsh 4-aryl-1,4-dihydropyridines using PEG400–Na2CO3 as an inexpensive catalyst system under solvent-free conditions
CaoYuqing, Mo Shulei, Zhang Zhan, Guo Yanxin, Li Yabin
(College of Pharmacy, Hebei University, Baoding 071002, China)
Abstract One-pot synthesis of the 4-aryl-1,4-dihydropyridine derivatives catalyzed by PEG400 -Na2CO3 combination system involving three component condensation reaction of an aromartic aldehyde,
b-ketoester, ammonium acetate under solvent free conditions to afford the corresponding products in good yields.Keywords 4-aryl-1,4-dihydropyridines, Hantzsch reaction, PEG400, solvent-free
1. INTRODUCTION
As green chemistry has become a major concern to organic chemists in present years,
reactions under solvent-free conditions have received much attention. These reactions
offer several advantages in preparative procedures such as environmentally friendly,
simplifying work-up, formation of cleaner products, enhanced selectivity and much improved
reaction rates [1].
Hantzsch 1,4-dihydropyridines (1,4-DHPS) are biologically active
compounds which including various vasodilator, antihypertensive, branchodilator,
antiatherodclerotic, hepatoprotective, antitu- mor, antimutagenic, geroprotective and
antidiabetic agents[2]. DHPs have found commercial utility as calcium channel
blochers [3, 4] such as Nifediine, Nitrendipine and Nimodipine. A number of DHP
calcium antagonists have been introduced as potential drugs for the treatment of
congestive heart failure [5, 6]. Among DHPs with other types of
bioactivity, cerebrocrast has been introduced as a neuroprotectant and cognition enhancer.
In addition, a number of DHPs with platelet antiaggregatory activity have also been
discovered[7].
1,4-dihydropyridines have been synthesized by the Hantzsch reaction[8],
which involves cyclocondensation of an aldehyde, b-ketoester, ammonia either in refluxing acetic acid or in refluxing
ethanol. 1,4-dihdropyridines have also been synthesized on a solid phase for making
combinatorial libraries[9]. Recently, Hantzsch's reaction for the
synthesis of dihydropyridines has received renewed interest and several improved
procedures have been reported[10-13]. However, there are several disadvantages
associated with these methodologies including unsatisfactory yields, long conversion
times, difficult handing of reagents, toxic organic solvents. Recently, the microwave
-promoting Hantzsch's reaction has also been reported[14-17]. Thus,
development of facile and environmental friendly synthetic methods to the Hantzsch's
reaction is demanded.
Polyethylenes glycols (PEGs) have been are known to function as
efficient phase transfer catalysts in a variety of organic reactions[18, 19].
PEGs are reported to have, at least in some cases efficiency comparable to that of crown
ethers to complex and transport alkali metal cations from the aqueous medium to the
organic phase. In addition to this, PEGs are nontoxic, thermally stable and inexpensive
compared to the conventional phase transfer catalyst (i.e. crown ethers or quaternary
ammonium salts). The reaction under solvent-free conditions has much attention in recent
times in the area of green synthesis. In the continuation of our investigation on the
research of using PEGs as phase transfer catalyst under solvent-free conditions[20].
In this article, we wish to report a mild and efficient version of the Hantzsch's reaction
for synthesis of 1,4-dihydropyridines using a catalyst amount of anhydrous Na2CO3
and PEG400 as an inexpensive catalyst system under solvent-free conditions.
Accordingly, treatment of benzaldehyde (1a), ethyl acetate (2) and ammonium
acetate (3) in the presence of 5% Na2CO3 and 5% of PEG400
resulted in the formation of 2,6-Dimethyl-3,5-Dicarboxylate-4-phenyl-1,4-dihydropyridine (4a)
in 85% yield (Scheme 1).
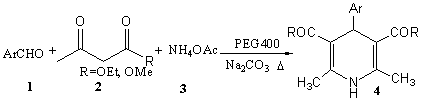
Scheme 1
2. RESULTS AND DISCUSSION
In our initial research, benzaldehyde was selected as a representative aldehyde in order
to optimize the reaction conditions for synthesis of 1,4-dihydropyridines in faster and
more efficient way. After some experimentation, we have found a set of conditions that
generally provide 1,4-dihydropyridines in good yields. The results showed that the
efficiency and the yield of the reaction in solution were much less than those obtained
under solvent-free conditions (Table 1). The molar ratios of benzaldehyde,
Table 1. 1,4-DHPS (4a) synthesis catalyzed by Na2CO3 in various solvents.a
Entry |
Solvent |
Temperature |
Na2CO3 (mol%) |
Time (h) |
Yield (%)b |
1 |
CH3CN |
Reflux |
5% |
3 |
60 |
2 |
CH2Cl2 |
Reflux |
5% |
4 |
80 |
3 |
DMF |
Reflux |
5% |
2 |
53 |
4 |
Toluene |
Reflux |
5% |
1.5 |
78 |
5 |
Benzene |
Reflux |
5% |
2 |
74 |
6 |
Solvent-free |
r.t |
5% |
4 |
84 |
7 |
Solvent-free |
80℃ |
1% |
2.5 |
82 |
8 |
Solvent-free |
80℃ |
5% |
1.5 |
88 |
9 |
Solvent-free |
80℃ |
10% |
1.5 |
87 |
a
Reaction conditions: benzaldehyde 1 (5mmol) ethyl atetoacetate 2 (10mmol) ammonium acetate 3 (5mmol).b isolated yield after crystallization.
Table 2. Synthesis of substituted 4-aryl-1,4-dihydropyridines using PEG400-Na2CO3 as an inexpensive catalyst system under solvent-free conditions. a
Entry |
Ar |
R |
Time |
Yield |
M.p |
|
4a |
C6H5 |
OEt |
90b |
88 |
156-157 |
158-160[12] |
4b |
4-CH3C6H4 |
OEt |
80 b |
86 |
135-137 |
136-138[12] |
4c |
3-NO2C6H4 |
OEt |
85c |
91 |
168-170 |
165-167[12] |
4d |
4-OCH3C6H4 |
OEt |
90b |
89 |
155-157 |
153-155[12] |
4e |
4-ClC6H4 |
OEt |
60 c |
90 |
144-147 |
143-146[12] |
4f |
4-NO2C6H4 |
OEt |
85 c |
95 |
128-130 |
128-129[12] |
4g |
4-OH-3-OMeC6H3 |
OEt |
90 c |
93 |
159-161 |
158-159[14] |
4h |
3,4-(CH3O)2C6H3 |
OEt |
90b |
86 |
138-139 |
138-140[14] |
4i |
4-OHC6H4 |
OEt |
70c |
94 |
225-228 |
222-224[14] |
4j |
2-NO2C6H4 |
OEt |
90 b |
85 |
171-173 |
|
4k |
C6H5CH=CH |
OEt |
85b |
87 |
148-150 |
149-150[15] |
4l |
C6H5 |
OMe |
80b |
86 |
196-198 |
196-198[16] |
4m |
4-OCH3C6H4 |
OMe |
90c |
88 |
185-186 |
186-188[16] |
4n |
4-OHC6H4 |
OMe |
60c |
92 |
231-232 |
230-232[16] |
4o |
4-ClC6H4 |
OMe |
75c |
89 |
195-197 |
196-198[16] |
4p |
4-CH3C6H4 |
OMe |
90b |
84 |
172-173 |
174-176[16] |
4q |
3-NO2C6H4 |
OMe |
80c |
91 |
211-213 |
210-212[16] |
4r |
2-ClC6H4 |
OMe |
80c |
88 |
196-198 |
194-196[16] |
4s |
C6H5CH=CH |
OMe |
90b |
87 |
177-178 |
176-178[16] |
a
Reaction conditions: aldehyde 1 (5mmol), b-ketoester 2 (10mmol), ammonium acetate 3 (5mmol), Na2CO3 (0.25mmol, 0.026g), PEG400 (0.25mmol, 0.1g).b The temperature were processed at 80oC.
c The temperature were processed at 100oC.
d Isolated yields.
In summary, we have described a mild and efficient protocol for the synthesis of 1,4-dihydro pyridines via Hantzsch's reaction of aromatic aldehyde with
b-ketoester and ammonium acetate using PEG400–Na2CO3 as an inexpensive catalyst system under solvent-free conditions. The simple experimental procedure combined with the facile catalyst makes this method quite simple, convenient and environmentally. This method not only provides a good yield in short time, but also avoids the use of organic solvent (cost, handing, safety and pollution). Hence, it is a useful addition to the existing methods.3.EXPERIMENTAL
1H-NMR spectra were obtained on a Bruker
AVANCE (400MHz) spectrometer using TMS as internal standard and CDCl3 as
solvent. IR spectra were recorded on a Bio-Rad FTS-40 spectrome ter
(KBr). TLC was GF254 thin layer chromatography with petroleum ether/ethyl acetate as
eluent. Aldehydes, b-ketoester and ammonium acetate were all commercial products and
were used without further purification. All liquid reagents were distilled before use.
Melting points were determined on a microscopy apparatus and are uncorrected.
General procedure for the synthesis of compounds
(4a-4s)
A mixture of aromatic aldehyde 1 (5mmol),
Compound (4a) 1HNMR (CDCl3): d: 1.24 (6H, t, J=7.2Hz, 2xCH3), 2.32 (6H, s, 2xCH3), 4.10 (4H, q, J=7.2Hz, 2x CH2O), 5.00 (1H, s, CH), 5.63 (1H, brs, NH), 7.12-7.31(5H, m, ArH); IR (KBr) n: 3341, 3060, 1688, 1651, 1488, 1372, 1229, 1211, 1123, 1091, 1020, 768, 703, 680 cm-1.
Compound (4b) 1HNMR(CDCl3): d: 1.24 (t, J=7.2Hz, 6H, 2xCH3), 2.27 (s, 3H, CH3), 2.32 (s 6H, 2xCH3), 4.10 (q, J=7.2Hz, 4H, 2x CH2O), 5.02 (s, 1H, CH), 5.60 (brs, 1H, NH), 7.10 (d, J=7.2Hz, 2H, ArH), 7.17 (d, J=7.2Hz, 2H, ArH); IR (KBr) n: 3350, 2990, 1700, 1649, 1490, 1390, 1200, 1100, 1090 cm-1.
Compound (4c) 1HNMR (CDCl3): d: 1.24 (6H, t, J=7.2Hz, 2xCH3), 2.36 (6H, s, 2xCH3), 4.10 (4H, q, J=7.2Hz, 2x CH2O), 5.10 (1H, s, CH), 5.65 (1H, brs, NH), 7.38 (1H, t, J=8.0Hz, ArH), 7.65 (1H, d, J=8.0Hz, ArH), 8.01 (1H, d, J=8.0Hz, ArH), 8.14 (1H, s, ArH). IR (KBr) n: 3358, 3093, 2987, 2960, 1696, 1651, 1603, 1507, 1489, 1372, 1300, 1245, 1210, 1166, 1123, 1090, 1018, 856, 786, 755, 695 cm-1.
Compound (4d) 1HNMR (CDCl3): (CDCl3): d: 1.24 (t, J=7.2Hz, 6H, 2xCH3), 2.33 (s 6H, 2xCH3), 3.77 (s, 3H, CH3O), 4.10 (q, J=7.2Hz, 4H, 2x CH2O), 5.02 (s, 1H, CH), 5.66 (brs, 1H, NH), 6.76 (d, J=8.2Hz, 2H, ArH), 7.21 (d, J=8.2Hz, 2H, ArH); IR (KBr) n: 3350, 2990, 1700, 1650, 1500, 1380, 1210, 834, 786, 750 cm-1.
Compound (4e) 1HNMR(CDCl 3): d:1.24 (t, J=7.2Hz, 6H, 2xCH3), 2.33 (s, 6H, 2xCH3), 4.10 (q, J=7.2Hz, 4H, 2x CH2O), 5.02 (s, 1H, CH), 5.59 (brs, 1H, NH), 7.13 (d, J=8.2Hz, 2H, ArH), 7.23 (d, J=8.2Hz, 2H, ArH); IR (KBr) n: 3358, 2987, 1695, 1651, 1507, 1489, 1372, 1210, 1123, 1090, 1018, 856, 786, 755, 695 cm-1.
Compound (4g) 1HNMR (CDCl3): d: 1.24 (6H, t, J=7.2Hz, 2xCH3), 2.32 (6H, s, 2xCH3), 3.83 (3H, s, OCH3), 4.10 (4H, q, J=7.2Hz, 2x CH2O), 4.90 (1H, s, CH), 5.48 (1H, s, OH), 5.55 (1H, brs, NH), 6.68 (1H, d, J=8.2Hz, ArH), 6.80 (1H, dd, J=8.2Hz, 2.0Hz, ArH), 6.84 (1H, d, J=2.0Hz, ArH); IR (KBr) n: 3351, 2983, 2953, 1681, 1653, 1598, 1514, 1489, 1370, 1303, 1272, 1218, 1160, 1122, 1095, 1020, 859, 800, 753 cm-1.
Compound (4i) 1HNMR(CDCl3): d:1.24 (6H, t, J=7.2Hz, 2xCH3), 2.32 (6H, s, 2xCH3), 4.10 (4H, q, J=7.2Hz, 2x CH2O), 5.02 (1H, s, CH), 5.40 (1H, s, OH), 5.49 (1H, brs, NH), 6.69(2H, d, J=8.2Hz, ArH), 6.89 (2H, d, J=8.2Hz, ArH); IR (KBr) n: 3347, 2987, 2939, 1660, 1634, 1511, 1488, 1369, 1316, 1227, 1171, 1022, 856, 845, 761 cm-1.
Compound (4l) 1HNMR (CDCl3): d: 2.32 (6H, s, 2xCH3), 3.64 (6H, s, 2xCH3OCO), 5.02 (1H, s, CH), 5.60 (1H, brs, NH), 7.10-7.29 (5H, m, ArH); IR (KBr) n: 3343, 3081, 3026, 2950, 1699, 1649, 768, 703, 680 cm-1.
Compound (4p) 1HNMR (CDCl3): d: 2.27 (3H, s, CH3), 2.32 (6H, s, 2xCH3), 3.64 (6H, s, 2xCH3OCO), 5.02 (1H, s, CH), 5.60 (1H, brs, NH), 7.10 (2H, d, J=7.2Hz, ArH), 7.17 (2H, d, J=7.2Hz, ArH); IR (KBr) v: 3314, 3105, 2942, 1697, 1655, 1495cm-1.
Compound (4q) 1HNMR (CDCl3): d: 2.36 (6H, s, 2xCH3), 3.64 (6H, s, 2xCH3OCO), 5.10 (1H, s, CH), 5.65 (1H, brs, NH), 7.38 (1H, t, J=8.0Hz, ArH), 7.65 (1H, d, J=8.0Hz, ArH), 8.01 (1H, d, J=8.0Hz, ArH), 8.14 (1H, s, ArH). IR (KBr) n: 3358, 3003, 2960, 1705, 1651, 1527, 1090, 1018, 856, 786, 755, 695 cm-1.
REFERENCES
[1] Tanaka K, Toda F. Chem. Rev, 2000, 100: 1025.
[2] Gaudio A C, Korolkovas A, Takahata Y. J. Pharm. Sci, 1994, 83: 1110-1115.
[3] Bocker R H, Guengerich F P. J. Med. Chem, 1986, 28: 1596.
[4] Gordeev M F, Patel D V, Gordon E M. J. Org. Chem, 1996, 61: 924-928.
[5] Sunkel C E, Santos L, Garcia A G, et al. J. Med. Chem,
1992, 35: 2407.
[6] Vo D, Matowe W C, Ramesh M, et al. J. Med. Chem, 1995, 38: 2851-2859.
[7] Cooper K, Fray M J, Parry M J, et al. J. Med. Chem, 1992, 35: 3115-3129.
[8] Hantzsch A. Chem, 1882, 1: 251.
[9] (a) Gordeev M F, Patel D V, Wu J, et al. Tetrahedron. Lett, 1996, 37: 4643-4646;
(b) Breitenbucher J G, Figliozzi G. Tetrahedron Lett, 2000,
41: 4311-4315.
[10] Sabitha G, Kiran G S, Srinivas C S, et al. Tetrhedron.Lett, 2003, 44: 4129-4131.
[11] Shi D Q, Mou J, Zhuang Q Y, et al. (Chin) J. Org. Chem, 2004, 24 (9): 1042-1044.
[12] Wang G W, Xia J J, Miao C B, et al. Bull. Chem. Soc. Japan, 2006, 79 (3):
454-459.
[13] Tu S J, Gao Y, Yu C X, et al. (Chin) J. Org. Chem, 2002, 22 (4): 269-271.
[14] Yadav J S, Reddy B V S, Basak A K, et al. Green Chem, 2003, 5: 60-63.
[15] Salehi H, Guo Q X. Synth. Commun, 2004, 34: 4349-4357.
[16] Sivamurugan V, Vinu A, Palnichamy M, et al. Heteroatom. Chem, 2006, 17 (4): 267-271.
[17] Heldebrant D J, Jessop P G. J. Am. Chem. Soc,
2003, 125: 5600-5601.
[18] Namboodiri V V, Varma R S. Green. Chem, 2001, 3: 146-148.
[19] Leadbeater N E, Marco M, Tominack B J. Org. Lett, 2003, 5 (21): 3919-3922.
[20] (a) Cao Y Q, Dai Z, Zhang R, et al. Synth. Commun, 2004, 34: 2965-2967;
(b) Cao Y Q, Dai Z, Zhang R. Synth. Commun, 2005,
35: 1045-1049.
曹玉庆,墨树磊,张占,郭艳欣,李亚彬
(河北大学药学院,071002,保定)
摘要 在无溶剂条件下,以芳香醛,β-酮酸酯,醋酸铵三组分为原料在聚乙二醇400和无水碳酸钠组成的体系催化下一锅法合成了4-芳基-1,4-二氢吡啶衍生物,得到了较好的收率(84-95%)。
关键词 4-芳基-1,4-二氢吡啶,Hantzsch反应,聚乙二醇400,无溶剂