http://www.chemistrymag.org/cji/2008/106028ne.htm |
Jun. 1,
2008 Vol.10 No.6 P.28 Copyright |
Tao Xueping, Sun Hongmei, Shen Qi, Zhang Yong
(Key Laboratory of Organic Synthesis of Jiangsu Province, Department of Chemistry and
Chemical Engineering, Suzhou University, Suzhou 215123, China)
Abstract The complex
[Ph-N=C(Me)-C(Me)=N-Ph]FeBr2 (1) has been synthesized via the reaction
of FeBr2 with equivalent of Ph-N=C(Me)-C(Me)=N-Ph in THF at room temperature in
ca. 93% yield, supported by elemental analysis and X-ray crystal determination. The center
iron atom is coordinated to two nitrogen atoms of a-diimine ligands and two bromine atoms to form a distorted
tetrahedral geometry.
Keywords Iron complex, a-Diimine ligand, Crystal structure, Synthesis
Received March 16, 2008; Supported by National Natural Science Foundation of China (No. 20772089) and the Key Laboratory of Organic Synthesis of Jiangsu Province.
1. INTRODUCTION
During the past few decades, a-diimine derivatives are among the most versatile and thoroughly
studied groups of ligands in late transition metal-based organometallic chemistry.[1,
2] Particularly, iron-based complexes bearing a-diimine ligands have recently drawn much attention in atom
transfer radical polymerization (ATRP)[3]. Up to date, a series of a-diimine Fe(II) complexes of (N,N)FeX2
(where [N,N] = RN=C(R')-C(R')=NR, R = aryl and alkyl, R'= H and alkyl) have been
synthesized, structural characterization and used as catalysts for ATRP of styrene[3,4,6,7,8]
and methyl methacrylate[3,5]. The substituent on the nitrogen atom of a-diimine ligands exerted great
influence on the catalytic behaviors of those iron complexes. For example, the bulky
arylimine stabilized iron catalysts, i.e. R= 2,6-diisopropylphenyl and mesityl,
gave rise to the catalytic chain transfer polymerization (CCT) of styrene, whereas the
alkylimine derivatives afforded ATRP of styrene[3]. It is suggested that a
steric effect could be used as the switch in the polymerization mechanism from CCT to
ATRP, and less crowded coordination sphere around the iron atom was of benefit to the ATRP
mechanism. With a view to gaining a detailed understanding of the steric effect of a-diimine ligands, herein we synthesized
and structural characterized a novel a-diimine Fe(II) complex of [Ph-N=C(Me)-C(Me)=N-Ph]FeBr2
(1), which lacks the ortho substituent on the aryl ring of a-diimine ligand. Very recently, a
analogue iron complex of [Ph-N=C(Me)-C(Me)=N-Ph]FeBr2 has been reported in
brief as a catalyst for the ATRP of styrene without X-ray structure analysis[3].
2. EXPERIMENTAL
2.1. General procedures
All manipulations were performed under pure argon with rigorous exclusion of air and
moisture using standard Schlenk techniques. Solvents were distilled from Na/benzophenone
ketyl under pure argon prior to use. Ph-N=C(Me)-C(Me)=N-Ph[9] was prepared
according to literature procedure. Elemental analysis was performed by direct combustion
on a Carlo-Erba EA-1110 instrument.
2.2. Synthesis of [Ph-N=C(Me)-C(Me)=N-Ph]FeBr2 (1)
By the modified literature procedure[10], a 100 mL round-bottomed flask was
charged with 1.20 g (5.1 mmol) of Ph-N=C(Me)-C(Me)=N-Ph and 60 mL of THF. With stirring,
0.84 g (3.9 mmol) of FeBr2 was added at 25ºC. The dark green
suspension was stirred for 18 h and filtered through Celite, and the resulting solid was
extracted with an additional 100 mL of THF. The solvent was removed in vacuo, and the
residue was washed with toluene. Removal of the toluene in vacuo afforded the product as
brown solid in yield of 93% (2.17 g). Anal. Calcd for C16H16Br2FeN2:
C, 42.52; H, 3.57; N, 6.20. Found: C, 42.89; H, 3.18; N, 6.59.
2.3 X-ray crystallography
Crystalline sample of 1 was grown from THF at –10ºC under Ar atmosphere. A dark-red needle crystal with dimension of
0.48 mm × 0.40 mm × 0.30 mm was mounted in a thin-walled glass capillary for X-ray
structural analysis. Diffraction data were collected on a standard Bruker-axs SMART CCD
area detector equipped with a graphite-monochromatized MoKa
radiation (l= 0.71073 Å) at 223(2) K using the w
scan mode in the range of 3.08 <q< 25.33°, and a multi-scan
absorption was applied to the intensity data. A total of 15716 reflections were collected,
of which 3136 (Rint = 0.0594) were independent and used. The frame was
integrated with the Bruker-axs SAINT program. Data were corrected for absorption with the
SADABS program. The structure was solved by direct methods and refined by full-matrix
least-squares procedures based on F2 (Bruker-axs, SHELXTL). All
non-hydrogen atoms were refined with anisotropic displacement coefficients. Hydrogen
atoms were determined with theoretical calculation and refined isotropically. The
structures were solved and refined using SHELXS-97 (Sheldrick, 1990) and SHELXL-97
(Sheldrick, 1997) programs, respectively. The final R = 0.0719 and wR =
0.1386 (w = 1/[s2(Fo2)
+ (0.0741P)2 + 0.0000P], where P = (Fo2
+ 2Fc2)/3 for 2761 observed reflections with I > 2s(I). S = 1.082, (D /s)max = 0.000, (Dr)max = 0.912 and (Dr)min = -0.853 e/Å.
3. RESULTS AND DISCUSSION
[Ph-N=C(Me)-C(Me)=N-Ph]FeBr2 (1) was readily synthesized via a modified
method by Dieck[10]. Thus, the treatment of FeBr2 with
Ph-N=C(Me)-C(Me)=N-Ph in THF (Scheme 1) resulted the target product 1 in high
yields. The complex 1 is isolated as air stable microcrystallines. Crystals of 1
suitable for an X-ray structure determination were grown from THF at -10ºC.
Scheme 1
The proposed structure
for 1 was first supported by elemental analysis. The further characterization for
its molecular structure was obtained by X-ray analysis.
The structure of the title compound is shown in Fig. 1. The
crystallographic data and analysis parameters are shown in Table 1. Selected bond lengths
and angles are listed in Table 2.
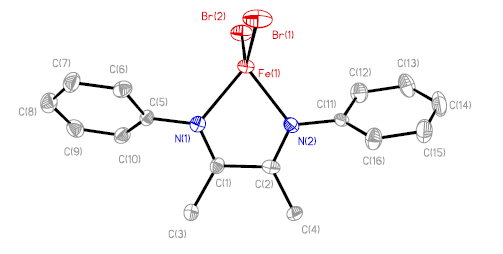
Fig. 1. Molecular structure of the title complex (1)
Table 1 Crystal data and structure analysis parameters
Complex |
LFeBr2 |
Dc (g/cm3) |
1.734 |
Crystal size (mm3) |
0.90×0.10×0.10 |
F(000) |
888 |
Empirical formula |
C16H16FeN2Br2 |
Scan mode |
w-2q |
Temperature (K) |
223(2) |
q (°) |
0.997 -25.33 |
Wavelength (Å) |
0.71070 |
Absorption
coefficient |
5.480 |
Formula weight |
451.98 |
Reflections collected |
15716 |
Crystal system |
Orthorhombic |
Independent reflections |
3136 |
Space group |
P21 |
Goodness-of-fit |
1.082 |
a (Å) |
8.267(3) |
R [I>2s (I)] |
0.0594 |
b (Å) |
10.975(3) |
wR2 |
0.1386 |
c (Å) |
19.086(6) |
Maximum diff. peak (e/Å3) |
0.912 |
V(Å3) |
1731.6(9) |
Minimum diff. peak (e/Å3) |
-0.853 |
Z |
4 |
Table 2 Selected bond lengths (Å) and bond angles (º)
Bond lengths |
|||
Fe (1)-Br(1) |
2.365(1) |
N (2)-C(2) |
1.307(9) |
Fe (1)-Br(2) |
2.364(1) |
N(2)-C(11) |
1.412(9) |
Fe (1)-N(1) |
2.106(6) |
C(1)-C(2) |
1.506(1) |
Fe (1)-N(2) |
2.082(6) |
C(1)-C(3) |
1.489(1) |
N(1)- C(1) |
1.286(8) |
C(2)-C(4) |
1.471(9) |
N (1)-C(5) |
1.431(9) |
C(5)-C(6) |
1.374(1) |
Bond Angles |
|||
Br(1)-Fe(1)-Br(2) |
123.4(5) |
N(1)-Fe(1)-N(2) |
77.6(2) |
N(2)-Fe(1)-Br(2) |
108.3(2) |
N(2)-Fe(1)-Br(1) |
112.86(2) |
X-ray structure analysis
reveals that the title complex crystallized in orthorhombic system and has a monomeric
structure in P212121 space group. This complex has no crystallographic symmetry
factor due to the torsional twist of the two Ph rings from each other. The dihedral angle
between the two Ph rings is ca. 22º. The fact that complex 1 being unsymmetry is quite
different from those of reported bulky arylimine derivatives, i.e. Cs
symmetry for 2,6-diisopropylphenylimine[5] or mesitylimine[3]
derivatives. This difference might be due to the lack of the ortho substituent on the
phenyl ring in 1, which leading to a more labile N-Cphenyl bond to form
a less crowded array of the two Ph rings in 1. The iron atom is bonded to two
nitrogen atoms of a-diimine
ligands and two bromine atoms to form a distorted tetrahedral geometry [angles in the
range of 77.6(2)-123.38(5)º] with the FeBr2 and FeN2 planes being nearly
orthogonal (ca. 86º) to each other. The five-membered chelate ring was essentially
planar (to within 0.005 Å), which is similar to tert-butylimine derivative[4],
but quite different to the 2,6-diisopropylphenylimine derivative (the chelate
ring is folded about the N···N vector by ca. 20°, the iron atom
lying 0.58 Å out of the C2N2
plane) [5]. The coplanarity of the iron atom with the chelate ring is expected
to give rise to an open geometry around the metal center[5].
The relatively short C=N bond lengths of 1.286(8) and 1.307(9) Å
and the relatively long C1-C2 bond lengths of 1.506(1) Å indicate that there is no
noticeable bond delocalization in the five-membered chelate ring of complex 1. This
pattern is very similar to those observed in the the tert-butylimine derivative[4]
and bulky arylimine derivatives[3, 5]. Besides, the bond lengths observed
here, including those to the iron atom, i.e. the Fe-N bond and the Fe-Br bond, are
very close to the reported values[3,5]. For example, the Fe-N bond lengths of
2.082(6) and 2.106(6) Å almost fall in the range previously reported values of
2.098(8)-2.132(8) Å presented in 2,6-diisopropylphenylimine,[5] mesitylimine[3]
and tert-butylimine[3] derivatives. The Fe-Br bond lengths of 2.365(1)
and 2.364(1) Å are almost the same as these values of 2.367(1) Å present in tert-butylimine
analogue[3].
In conclusion, the present work provides an opportunity to examine the
steric constraints around the iron atom by comparison of the structural data of 1
with its bulky arylimine derivatives or tert-butylimine analogue. The X-ray
structure analysis reveals that the present modification in the ortho substituent on the
aryl ring has an obvious effect on the geometry around the iron atom although exerts a
little effect on the relative bond lengths presented in 1. The lack of ortho
substituent on the phenyl ring leads a more open geometry around the iron atom, which is
expected to make the center metal more accessible and allow an ATRP to dominate[5].
4. CONCLUTION
In summary, we have successfully synthesized a new a-diimine Fe(II) complex [Ph-N=C(Me)-C(Me)=N-Ph]FeBr2 1
in high yield, and characterized its structural feature by X-ray crystallography. In
comparison with the reported results[3,4,6,7,8], the present work provides a
direct evidence to elucidate that the no substituent on the aryl ring of a-diimine ligands make a little
difference on the molecular structure of the a-diimine-based iron complexes. The further investigation focus on
its catalytic activity and modification is proceeding in our laboratory.
REFERENCES
[1] Ittel, S. D.; Johnson, L. K.; Brookhart, M.. Chem. Rev. 2000, 100: 1169.
[2] Gibson, V. G.; Spitzmesser, S. K. Chem. Rev. 2003, 103: 283.
[3] O'Reilly, R. K.; Shaver, M. P.; Gibson, V. C.; White, A. J. P. Organometallics 2007,
40: 7441, and the references cited therein.
[4] Gibson, V. C.; O’Reilly, R. K.; Reed, W.; Wass,
D. F.; White,A. J. P.; Williams, D. J. Chem.Commun. 2002, 1850.
[5] Gibson, V. C.; O’Reilly, R. K.; Wass, D. F.;
White,A. J. P.; Williams, D. J. Macromolecules 2003, 36: 2591.
[6] Shaver, M. P.; Laura, E. N.; Allan, H. S.; Gibson, V. C. Angew. Chem. 2006, 118: 1263.
[7] Shaver, M. P.; Laura, E. N.; Allan, H. S.; Gibson, V. C. Organometallics 2007, 26:
4725.
[8] Laura, E. N.; Allan, H. S.; Shaver, M. P.; Andrew J. P.; White.; Gibson, V. C. Inorg.
Chem. 2007, 46: 8963.
[9] Tempel, D. J.; Johnson, L. K.; Huff, R. L.; White, P. S.; Brookhart, M. J. Am. Chem.
Soc. 2000, 122: 6686.
[10] Tom Dieck, H.; Diercks, R. Angew. Chem., Int. Ed. Engl. 1983, 22: 778.
陶雪平,孙宏枚,沈琪,张勇
(江苏省有机合成重点实验室,苏州大学化学化工学院,苏州 215123,中国)
摘要 通过a-二亚胺和FeBr2的1:1(摩尔比)反应,我们以高收率合成了a-二亚胺铁配合物1。配合物1通过了元素分析和X-衍射等表征。结构测定表明,中心金属原子Fe与a-二亚胺上的两个氮原子和两个溴原子配位,形成了一个略扭曲的四面体。本文的研究结果表明a-二亚胺芳基上的取代基对相应铁配合物的组成和结构有一定的影响。
关键词 铁配合物、a-二亚胺、晶体结构、合成