http://www.chemistrymag.org/cji/2008/106029pe.htm |
Jun. 1,
2008 Vol.10 No.6 P.29 Copyright |
Zhu Li a, Jia Jinliang a,
Yang Xuwub, Gao Shenglib
(
Received March 12, 2008; South China of Agricultural University for financial Support (4900K07283)
Abstract Thirteen solid complexes were synthesized with sodium diethyldithiocarbamate (NaEt2dtc·3H2O), 1,10-phenanthroline (o-phen·H2O) and hydrated lanthanide chlorides in absolute ethanol, which are identified as a general formula of RE(Et2dtc)3(phen) (RE=La, Pr, Nd, Sm~Lu). IR results revealed that two sulfur atoms in NaEt2dtc and two nitrogen atoms in o-phen coordinate to RE3+ in bidentate fashion, respectively. UV spectrum of the complexes suggested energy transfer between o-phen and RE3+ is the primary process, and the main absorbtion peaks showed a slight red shift than that of o-phen. The constant-volume combustion energies of complexes were determined by a precise rotating-bomb calorimeter at 298.15K. The standard enthalpies of combustion and standard enthalpies of formation were calculated for these complexes, respectively. The experiment results showed "triplet effect" of rare earth.Keywords rare earth sulfocompound; constant-volume combustion energy; standard enthalpy of combustion; standard enthalpy of formation; triplet effect
The series of complexes containing lanthanide
sulfide have well biological activity, the usage of them as antiseptic and insecticide
have been attended widely[1]. they also could be used as the precursors of
ceramics and thin film materials[2], grease additive and accelerant of
vulcanizing rubber[3]. Preparation, spectroscopic properties and the crystal
structure of some complexes had been reported [4-5].
Thermodynamic data could offer better interpretation to the essence of
lanthanide-sulfide bonds and stability of this series of complexes, and part investigation
has been carried out concerning the thermochemical properties for these complexes[6-7].In
this paper, constant–volume combustion energy of
thirteen complexes have been determined, the standard enthalpies of combustion and
standard enthalpies of formation have been calculated on the basis of the constant-volume
combustion energies of the complexes, respectively. The gained thermodynamic quantities
presented the "triplet effect" of rare
earth, suggesting that a certain amount of covalence is present in the chemical bonds of
the complexes.
1 EXPERIMENTAL
1.1 Reagents
Lanthanide chloride hydrate, NaEt2dtc
1.2 Preparation and composition of the complexes
The methods of preparation for the complexes are the same as those in Ref.[6-7]. RE3+ was determined with EDTA by complexometric titration; C, H, N and S analyses were carried out by Vario EL III CHNOS of German. The final results are showed in Table 1. they are identified as a general formula of RE(Et2dtc)3(phen).
1.3 Apparatus and experimental conditions
The constant-volume combustion energies of the complexes were determined by a precise rotating-bomb calorimeter (RBC-type II), The main experimental procedures and structure were described previously[8]. The correct value of the heat exchange was calculated according to Linio-Pyfengdelel-Wsava formula[9].The initial temperature was regulated to (25.0000 ± 0.0005) ℃, and the initial oxygen pressure was 2.5MPa. The digital indicator for temperature measurement was used to promote the precision and accuracy of the experiment.
The calorimeter was calibrated with benzoic acid of 99.999 % purity, which has an isothermal heat of combustion of -26434 J·g-1 at 25℃, and the experimental result were (17775.09 ± 7.43) J·K–1 (Table 2), the precision was 4.18×10-4. To determine the standard combustion energies of sulfur-containing compounds, the constant-volume combustion energy of thianthrene has been determined as being (-33507.76 ± 14.13) J·g–1 (Table 2) ,which is in good agreement with (-33468 ± 4 ) J·g–1 [10]. The precision and the accuracy were 4.22×10-4 and 1.19×10-3, respectively, the calorimetric system is accurate and reliable.
The analytical methods of final products (gas, liquid and solid) were the same as these in Ref.[8], the analytical results of the final products indicated that the combustion reactions were complete.
Table 1 Analytical results related to the compositions of the complexes (%)
Sample |
RE |
S |
C |
N |
H |
La(Et2dtc)3(phen) |
18.21(18.18) |
42.53(42.45) |
9.09(9.17) |
25.07(25.18) |
4.87(5.01) |
Pr(Et2dtc)3(phen) |
18.30(18.40) |
42.23(42.36) |
9.10(9.14) |
25.07(25.12) |
4.79(5.00) |
Nd(Et2dtc)3(phen) |
18.59(18.75) |
42.08(42.16) |
9.01(9.10) |
24.87(25.01) |
4.69(4.98) |
Sm(Et2dtc)3(phen) |
19.42(19.39) |
41.81(41.82) |
9.00(9.03) |
24.79(24.81) |
4.97(4.94) |
Eu(Et2dtc)3(phen) |
19.57(19.56) |
41.72(41.74) |
9.04(9.01) |
24.77(24.76) |
4.89(4.93) |
Gd(Et2dtc)3(phen) |
20.12(20.10) |
41.44(41.46) |
8.97(8.95) |
24.56(24.59) |
4.91(4.90) |
Tb(Et2dtc)3(phen) |
20.28(20.27) |
41.29(41.37) |
8.99(8.93) |
24.52(24.54) |
4.92(4.89) |
Dy(Et2dtc)3(phen) |
20.85(20.63) |
40.77(41.18) |
8.80(8.89) |
24.89(24.43) |
4.68(4.86) |
Ho(Et2dtc)3(phen) |
20.99(20.88) |
24.82(24.36) |
40.82(41.05) |
8.81(8.86) |
4.57(4.67) |
Er(Et2dtc)3(phen) |
20.30(20.27) |
24.50(24.54) |
41.26(41.37) |
8.99(8.93) |
4.95(4.89) |
Tm(Et2dtc)3(phen) |
21.10(21.28) |
24.32(24.23) |
40.67(40.85) |
8.89(8.82) |
4.76(4.82) |
Yb(Et2dtc)3(phen) |
21.47(21.68) |
24.18(24.11) |
40.81(40.64) |
8.85(8.78) |
4.68(4.80) |
Lu(Et2dtc)3(phen) |
22.01(21.87) |
24.10(24.05) |
40.65(40.54) |
8.64(8.75) |
4.64(4.79) |
a
The data in brackets are calculated values.Table 2 Experimental results for the combustion energies of benzoic acid and thianthrene
Samples |
Mass of complexes |
Calibrated heat of combustion wire qc/J |
Calibrated heat of acid |
Calibrated |
Combustion energies of complexes |
0.99702,0.78940 |
10.35, 8.10 |
24.78, 20.89 |
1.4834, 1.1746 |
17790.45, 17789.88 |
|
benzoic acid |
0.83060, 0.96869 |
12.60,12.60 |
20.43, 17.43 |
1.2382, 1.4418 |
17758.93, 17780.82 |
0.99485, 0.90036 |
12.60, 9.28 |
20.80, 21.67 |
1.4800, 1.3429 |
17798.18, 17745.97 |
|
mean±SD |
17775.09±7.43 |
||||
0.48860, 0.48886 |
12.60, 11.70 |
1383.69, 1384.41 |
0.9998, 1.0015 |
33514.62, 33558.98 |
|
thianthrene |
0.49011, 0.48798 |
12.60, 12.60 |
1387.90, 1381.96 |
1.0028, 0.9977 |
33511.58, 33484.26 |
0.48835, 0.48823 |
12.60, 12.60 |
1382.99, 1382.65 |
0.9977, 0.9992 |
33456.78, 33520.31 |
|
mean±SD |
33507.76±14.13 |
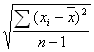
2 RESULTS AND DISCUSS
2.1 IR spectra of the complexes
IR spectra of the complexes are similar because of their similar structure[11,12]:
the peaks of about 3340 cm-1 are assigned to the characteristic absorption of
hydroxyl group in hydrated lanthanide chlorides and ligands, which is not present in the
complexes , showing that the complexes does not contain water. The skeleton vibration and
the
(i) (ii) (iii)
2.2 UV spectra of the complexes
The UV spectra of the complexes and ligands were determined by a instrument of Perkielmer Lambda 40 of America, the lmax were listed in Table 3. Intraligand transition (p→p*, n→p*) were displayed at about 329.56nm and 380.12nm for the free ligand NaEt2dtc·3H2O [14], and characteristic transition (p→p*) of o-phen was present at 265.05 nm[15]. As for the complexes, the absorption peaks were well similar, whether shape, absorbency or place of peaks (Fig.1), which demonstrate that energy transfer between o-phen and RE3+ is the primary process[15], and strong absorption of o-phen fully or partly shield that of NaEt2dtc·3H2O in the complexes[17]. In addition, the main absorption peaks of the complexes show a slight red shift than that of o-phen, which was due to pelectrons of nitrogen atoms of o-phen have excursion to the 5d blank orbits of RE3+, and resulting to the increment of p electron conjugate action for the whole system, when s-coordinate band of the complexes were formed between nitrogen atoms of o-phen and RE 3+.
Table 3 The main UV and IR absorbtion data of the ligands and the complexes
Ligands and complexes |
nOH | nC═C | nC─H | nCN | nCSS |
|
lmax |
| LnCl3·nH2O (n<4) | 3349-3393 |
||||||
o-phen ·H2O |
3388 |
1617,1587, 1561, 1504 |
854, 739 |
265.05 |
|||
NaEt2dtc ·3 H2O |
3366 |
1477 |
986 |
|
|||
La(Et2dtc)3(phen) |
1624, 1588, 1572, 1516 |
848, 729 |
1480, 1516 |
995 |
9 |
266.59 |
|
Pr(Et2dtc)3(phen) |
1622, 1589, 1570, 1515 |
851, 730 |
1480,1515 |
997 |
11 |
266.93 |
|
Nd(Et2dtc)3(phen) |
1624, 1589, 1572, 1516 |
851, 730 |
1482, 1516 |
997 |
11 |
267.26 |
|
Sm(Et2dtc)3(phen) |
1623, 1589, 1571, 1516 |
852, 730 |
1481, 1516 |
995 |
9 |
267.77 |
|
Eu(Et2dtc)3(phen) |
1624, 1589, 1572, 1516 |
852, 730 |
1482, 1516 |
1002 |
16 |
267.49 |
|
Gd(Et2dtc)3(phen) |
1624, 1589, 1572, 1516 |
852, 730 |
1481, 1516 |
1003 |
17 |
267.94 |
|
Tb(Et2dtc)3(phen) |
1624, 1589, 1572, 1516 |
852, 730 |
1482, 1516 |
1003 |
17 |
267.91 |
|
Dy(Et2dtc)3(phen) |
1624, 1589, 1572, 1517 |
853, 730 |
1482, 1517 |
1001 |
15 |
267.85 |
|
Ho(Et2dtc)3(phen) |
1625, 1589, 1572, 1517 |
853, 730 |
1482, 1517 |
1006 |
20 |
268.10 |
|
Er(Et2dtc)3(phen) |
1625, 1590, 1572, 1517 |
853, 730 |
1482, 1517 |
1007 |
21 |
267.30 |
|
Tm(Et2dtc)3(phen) |
1625, 1590, 1572, 1517 |
853, 730 |
1482, 1517 |
1007 |
21 |
268.39 |
|
Yb(Et2dtc)3(phen) |
1625, 1590, 1572, 1517 |
854, 730 |
1481, 1517 |
1006 |
20 |
267.95 |
|
Lu(Et2dtc)3(phen) |
1625, 1590, 1572, 1517 |
854, 730 |
1482, 1517 |
1004 |
18 |
267.89 |
|
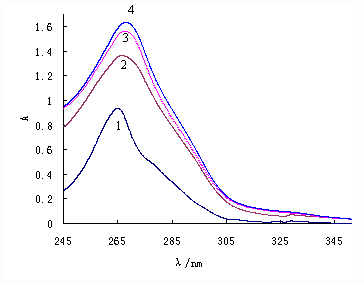
Figure 1 UV absorption curves of ligand o-phen(1)
and Complexes La(Et2dtc)3(phen)(2),Sm(Et2dtc)3(phen)(3)
and Lu(Et2dtc)3(phen)(4)
The methods of determination and calculation of the constant-volume combustion energies (
The standard combustion enthalpies of the complexes (
RE(Et2dtc)3(phen) (s) +
(RE=La, Pr, Nd, Sm~Lu)
The standard combustion enthalpies of the complexes are calculated by the following equations:
where ng is the total amount in mole of gases which present in products or in reactants, R = 8.314 J·K-1·mol-1, T = 298.15K.
The standard enthalpies of formation of the complexes (
![]() (RE(Et2dtc)3(phen), s) = [
(RE(Et2dtc)3(phen), s) = [![]()
![]() (RE2O3, s) + 27
(RE2O3, s) + 27![]() (CO2,
g) + 19
(CO2,
g) + 19![]() (H2O,l)
(H2O,l)
+ 6![]() (SO2, g) +
(SO2, g) +![]()
![]() (N2
, g)] -
(N2
, g)] -![]() (RE(Et2dtc)3(phen),
s) (4)
(RE(Et2dtc)3(phen),
s) (4)
where ![]() (RE2O3, s) = (-1793.14 ± 0.79) (La), (-1823.39 ±
6.69) (Pr), (-1808.12 ± 1.00) (Nd), (-1808.12 ±1.00) (Sm), (-1663.00 ± 1.62) (Eu),
(-1815.60 ± 3.60) (Gd), (-1827.6 ± 2.0) (Tb), (-1865.39 ± 3.89) (Dy), (-1880.92 ±
4.81) (Ho), (-1897.29 ± 4.88) (Er), (-1888.66 ± 5.86) (Tm), (-1814.50 ± 2.22) (Yb),
(-1881.96 ± 7.53) (Lu) kJ
(RE2O3, s) = (-1793.14 ± 0.79) (La), (-1823.39 ±
6.69) (Pr), (-1808.12 ± 1.00) (Nd), (-1808.12 ±1.00) (Sm), (-1663.00 ± 1.62) (Eu),
(-1815.60 ± 3.60) (Gd), (-1827.6 ± 2.0) (Tb), (-1865.39 ± 3.89) (Dy), (-1880.92 ±
4.81) (Ho), (-1897.29 ± 4.88) (Er), (-1888.66 ± 5.86) (Tm), (-1814.50 ± 2.22) (Yb),
(-1881.96 ± 7.53) (Lu) kJ
Table 4 Combustion energies, standard combustion enthalpies and standard enthalpies of formation of the complexes at 298.15K
Complexes |
|
|
|
La(Et2dtc)3(phen) |
-17455.98 ± 7.98 |
-17475.19 ± 7.98 |
-1257.78 ± 8.84 |
Pr(Et2dtc)3(phen) |
-17840.67 ± 10.38 |
-17859.88 ± 10.38 |
-888.22 ± 11.55 |
Nd(Et2dtc)3(phen) |
-18674.22 ± 8.33 |
-18693.43 ± 8.33 |
-47.03 ± 9.17 |
Sm(Et2dtc)3(phen) |
-17406.90 ± 9.69 |
-17426.11 ± 9.69 |
-1317.99 ± 10.45 |
Eu(Et2dtc)3(phen) |
-17410.63 ± 8.95 |
-17429.84 ± 8.95 |
-1238.06 ± 9.75 |
Gd(Et2dtc)3(phen) |
-18673.71 ± 8.15 |
-18692.92 ± 8.15 |
-51.28 ± 9.17 |
Tb(Et2dtc)3(phen) |
-17646.95 ± 8.64 |
-17666.16 ± 8.64 |
-1084.04 ± 9.49 |
Dy(Et2dtc)3(phen) |
-16730.21 ± 9.25 |
-16749.42 ± 9.25 |
-2019.68 ± 10.19 |
Ho(Et2dtc)3(phen) |
-18213.19± 8.18 |
-18232.40 ± 8.18 |
-544.46 ± 9.33 |
Er(Et2dtc)3(phen) |
-18436.62± 9.11 |
-18455.83 ± 9.11 |
-329.48 ± 9.91 |
Tm(Et2dtc)3(phen) |
-18161.61± 8.46 |
-18180.82 ± 8.46 |
-599.91 ± 9.73 |
Yb(Et2dtc)3(phen) |
-17954.08± 8.11 |
-17973.29 ± 8.11 |
-770.36 ± 9.02 |
Lu(Et2dtc)3(phen) |
-17898.22± 8.59 |
-17917.43 ± 8.59 |
-859.95 ± 10.12 |
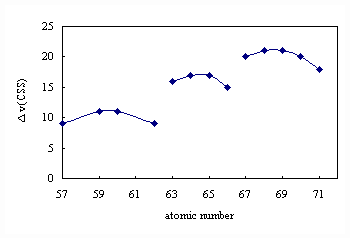
Figure 2 plot of Dn( CSS ) against the atomic number (Dn(CSS) is the
stretching vibration difference between diethyldithiocarbamate
and complexes )
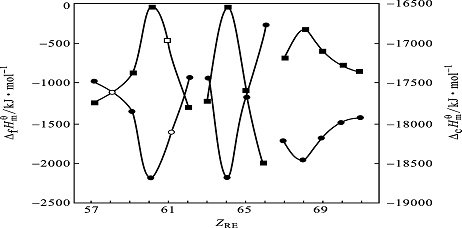
Figure 3 Plots of ![]() and
and ![]() against the atomic numbers (ZRE) of rare-earth for the complexes
against the atomic numbers (ZRE) of rare-earth for the complexes
●![]() ; ■
; ■![]()
3 CONCLUSIONS
![]() and
and ![]() of the complexes are plotted against the atomic numbers of the elements in
the lanthanide series, as showed in Fig.3. The curves show the "tripartite
effect" of rare earth, which is consistent with
the observed result in Fig.2, suggesting that a certain amount of covalence is present in
the chemical bonds between RE3+ and ligands that is caused by the incomplete
shield of 5s25p6 orbital to 4f electrons. The
experimental results is in good agreement with Nephelauxetic effect of 4f electrons of
rare earth.
of the complexes are plotted against the atomic numbers of the elements in
the lanthanide series, as showed in Fig.3. The curves show the "tripartite
effect" of rare earth, which is consistent with
the observed result in Fig.2, suggesting that a certain amount of covalence is present in
the chemical bonds between RE3+ and ligands that is caused by the incomplete
shield of 5s25p6 orbital to 4f electrons. The
experimental results is in good agreement with Nephelauxetic effect of 4f electrons of
rare earth.
On the basis of Fig.3, the
corresponding thermochemical data (standard combustion enthalpies, standard enthalpies of
formation ) of Ce(Et2dtc)3(phen) and Pm(Et2dtc)3(phen)
could be estimated.
REFERENCES
[1] Marinho E.P., Sousa W.S.C., Melo D.M.A. Thermochim. Acta. 2000, 344: 67.
[2] Ivanoy, R. A., Korsakoy, I. E., Kuzmina, N. P. et al., Mendeleev Commun. 2000, 3: 98.
[3] Zhang W G., Yin X., Fan J. et al., J. Rare Earth(Xitu Xuebao). 2004, 22 (3): 299.
[4] Su C. Y., Tan, M. Y., Tang, N. et al., J. Coord. Chem. 1996, 38 (3): 207.
[5] Zhou R, Sun Y H. J. Xinjiang Univ.(Xinjiang Daxue Xuebao). 1997, 14 (4): 67.
[6] Zhu L., Yang X.W., Chen S.P., et al., J. Inorg. Chem. 2004, 20 (11): 1303.
[7] Zhu L., Jiao B.J., Shuai Q. et al., Chin. J. Org. Chem. 2004, 24 (11): 1417.
[8] Yang, X. W., Chen, S. P., Gao, S. L. et al., Instrum. Sci. & Technol. 2002,
30 (3): 311.
[9] Popov, M. M., Thermometry and Calorimetry, Moscow: Moscow University Publishing House,
1954: 382.
[10] Marthada, V. K., Journal of Research of the National Bureau of Standard, 1980, 85
(6): 467.
[11] Dong Q.N. IR Spectroscopy(Hongwai Guangpu), Chemical and Industrial Press,
Beijing,1979, p.194.
[12] Nakamoto K.( Huang D.H., Wang R.Q Translated) Infrared and Raman Spectra of Inorganic
and Coordination Compounds(Wuji Peihewu de Hongwai ji Laman Guangpu). 4th Edn. Beijing:
Chemical Industriy Press, 1991.
[13] Nakamoto K., Fujita J., Condrate R. A. et al., Chem. Phys. 1963, 39: 423.
[14] Crosby G.A., Whan R.E., Alire R.M. J. Chem. Phys.1961,34 (3): 743.
[15] Yan B., Zhang H.J., Wang S.B. J. Rare Earth(Xitu Xuebao).1998, 16 (4): 375.
[16] Sokolov V.V., Kamarzin A.A., Trushnikova L.N. et al., J. Alloy. Comp.1995, 225: 567.
[17] Rederick, D. R., Experimental Therrnochemistry. Interscience Publishers Ltd., 1956:
88.
[18] Robert, C. W., CRC Handbook of Chemistry and Physics, Chemical Rubber Publishing
Company, 1977-1978, 55: D45.
稀土含硫配合物的恒容燃烧能测定及标准摩尔生成焓计算
朱丽a 贾金亮a 杨旭武b 高胜利b
(a华南农业大学 理学院 广东 广州 510642; b西北大学化学系 陕西省物理无机化学重点实验室 陕西
西安 710069)
摘要 在无水乙醇中,通过二乙基二硫代氨基甲酸钠(NaEt2dtc),1,10-邻菲咯啉(o-phen)和水合氯化稀土盐反应制备得到了13种稀土含硫固态配合物,其通式为RE(Et2dtc)3(phen) (RE=La, Pr, Nd, Sm~Lu)。配合物的红外光谱表明配体NaEt2dtc中的硫原子和o-phen中的氮原子均与RE3+双齿配位。紫外光谱显示在配合物中邻菲咯啉与稀土离子之间的能量传递为主要过程,且其最大吸收峰的位置相对邻菲咯啉的都有微小红移。在298.15K下,通过RBC- II型精密转动弹热量计测定了配合物的恒容燃烧能,并分别计算了标准摩尔燃烧焓及标准摩尔生成焓。所得实验结果显示出了稀土元素的“三分组效应”。
关键词 稀土含硫配合物; 恒容燃烧能;
标准摩尔燃烧焓;标准摩尔生成焓;三分组效应