http://www.chemistrymag.org/cji/2008/106031pe.htm |
Jun.
1, 2008 Vol.10 No.6 P.31 Copyright |
Hu Qiufen 1,2,3, Li Yinke 2,Yang Xianjun 3, Wei Qunyan 3, Huang Zhangjie 1,3
(1 Faculty of Materitals and Metallurgical Engineering, Kunming University of Science and Technology, Kunming 650031; 2 Department of Chemistry, Yunnan Institute of the Nationalities, Kunming 650031; 3 Department of Chemistry, Yunnan University, Kunming 650091)
Abstract A simple, sensitive and reproducible spectrophotometric method for the determination of etodolac was described. This method based on the etodolac can reduce Fe3+ to Fe2+ in the presence of 2,2'-bipyridyl (Bpy) and pH 3.5 ~ 6.0 acetate buffer medium. The Fe2+ can reacts with Bpy to form a Fe2+-Bpy colored complex. The maximum absorbance of the colored complex is at 500 nm. Beer
's law is obeyed in the range of 0.5 - 25 m g/mL for etodolac in this method. The method was applied to the determination of etodolac in tablets without any interference from common excipients. The relative standard deviations were £ 0.82% with recoveries 97% - 102%. Results are satisfactory.Keywords Etodolac; 2,2'-bipyridyl; Spectrophotometry
1. INTRODUCTION
Etodolac (ETD) is nonsteroidal
anti-inflamatory antirheumatic drugs [1]. A survey of the literature revealed
that there have been very few methods for the determination of ETD in biological fluids,
tablets and in presence of its enantiomer. The techniques used in this connection include
only HPLC, GC, spectrofluorimetric and spectrophotometric methods [2-4].
Extensive literature survey revealed that no method is available for determination of ETD
in pure form and tablets by redox reaction.
2,2
2. EXPERIMENTAL
2.1. Apparatus
A UV-160 A spectrophotometer (Shimidzu Corporation, Tokyo, Japan) equipped with 1 cm cells
was used for all absorbance measurements. The pH values were determined with a Beckman F-200 pH meter (Beckman Instruments,
Fullerton, CA, USA).
2.2. Materials and Reagents
All chemicals and materials were of
analytical grade and all solutions were freshly prepared in bidistilled water. Etodolac
(ETD) pure grade supplied by Fluka Corporation and its tablets (Napilac capsules, 200 mg
ETD/Capsule) and (Etodine capsules, 300 mg ETD/Capsule) was provided by Kunming Pharmacy
Corporation. Stock standard solution of ETD was prepared by dissolving 100 mg pure drug in
methanol and completed to 100 mL with the same solvent to obtain a standard solution of
1.0 mg/mL. Working solutions were prepared by an appropriate dilution of the stock
standard solution. The iron(III)- 2,2
2.3. Recommended Analytical Procedure
Transfer aliquots (0.05-2.5 mL) of standard solutions (100 m g/mL) in a series of 10 mL calibrated flasks. To which, 1.0 mL Fe3+-Bpy or reagent solutions and 4.0 mL acetate buffer solution of pH 4.5 were added. The mixture was heated on a water bath at 80°C for 10 min. Then, the mixture was cooled to room temperature (25 ± 1°C) and the volume was made up to the mark with bidistilled water. The colored complex formed was measured at 500 nm against a reagent blank treated similarly.
2.4. Analysis of etodolac in tablets
Ten etodolac tablets were accurately weighed and powdered. An accurately weighed quantity equivalent to 20 mg ETD was dissolved in 20 mL methanol and transferred to a 100 mL calibrated flask. The contents of the flask was shaken for 10 min, and then made up to the mark with methanol. The general procedure was then followed in the concentration ranges already mentioned above.
3. Results and Discussion
3.1. Absorption Spectrum
This method is based on the formation of Bpy-Fe2+ complex upon the reaction of ETD with the Fe3+-Bpy reagent. The reaction proceeds through the reduction of Fe3+ to Fe2+ and the subsequent formation of an intensive orange-red coloration of the complex. The absorption spectra of the colored complex species in the proposed methods at the optimum conditions was scanned in the double beam mode against a reagent blank in the range 400-600 nm and recorded in the general procedures show a characteristic l max at 500 nm (Fig.1).
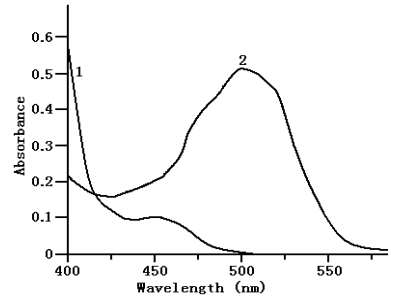
Fig.1 Absorption spectra
(1) Reagent blank against water
(2) Fe(III)-(2,2'-bipyridyl) with ETD (5.0 mg/mL) against reagent blank
3.2. Effect of Acidity
An acetate buffer solution was the optimal one of those examined (universal, phosphate,
thiel, borate and acetate). The pH adjustment is necessary especially in acidic medium
because the reaction was affected by the change of the pH in the range of (2.0 -6.0). The
optimum pH value was 2.6-5.5 for this method. A acetate buffer solutions of pH 4.5 was
recommended to control pH. As the use of 3.5 - 6.0 ml of the buffer solution (pH 4.5) was
found to give a maximum and constant absorbance. The use of 4.0 ml buffer solution was
recommended.
3.3. Effect of reagent concentration
The addition of 1.0 mL Fe3+-Bpy reagent solutions was sufficient to obtain the
maximum and reproducible absorbance for 20 m g/mL-1 of ETD. Smaller amounts
give incomplete complex formation. Whereas a larger concentration had no effect on complex
formation, although the absorbance increased slightly due to the background of the reagent
used. Accordingly, 1.0 ml of Fe3+-Bpy reagent solutions solution was added in
all further measurements.
3.4. Effect of Temperature and Heating Time
The effect of temperature and heating time on the formation of the colored complex were
studied. The reaction of ETD with the reagent proceeds very slowly at room temperature.
Higher temperature was used to accelerate the reaction. Maximum absorbance was obtained
after heating for about 10 min with Fe2+-Bpy colored complexes on a water bath
at 80
3.5. Calibration Curve and Sensitivity
The calibration curve shows that Beer's law is obeyed in the concentration range of 0.5 - 25 m g/mL. The linear regression equation obtained was: A = 0.00615 + 0.0572 C (r = 0.9999). The molar absorptivity was calculated to be 1.79×104 L.mol-1.cm-1. The detect limit, based on (LOD = 3s/k) is 0.06 m g/mL.
3.6. The method precision and recovery
The intra-day precision and inter-day precision were calculated from data obtained during a 7-day validation, solutions containing four different concentrations of ETD were prepared and analyzed in seven replicates. Precision of the assay was determined by repeatability (intraday) and intermediate precision (inter-day). To assess intraday variation (repeatability), calibration curve was prepared seven times on the same day. Intermediate precision was assessed by comparing the assays on different days (7 days, n = 7 at each concentration). The results shown that the relative standard derivation of overall intra-day variations were less than 0.68%, and the relative standard derivation of inter-day variations were less than 0.82 %. This method is high precision.
The recovery test of the proposed method was prepared by adding a known amount of standard at three different levels (1.0 m g/mL, 2.0 m g/mL, 8.0 m g/mL) to the pre-analysed sample. The results shown that the recoveries (n=7) were ranged from 97% - 102%. This method is high recovery.
3.7. Effects of Interference
The criterion of interference was an error of not more than ± 3.0% in the absorbance. To test the efficiency and selectivity of the proposed analytical method to tablets, a systematic study of additives and excipients (e.g. lactose, glucose, dextrose, talc, calcium hydrogen phosphate, magnesium stearate and starch) that usually present in dosage forms. Experimental showed that there was no interference from additives or excipients for the examined method as shown in Table 1.
Table 1. Determination of ETD in presence of additives or excipients
Material |
Amount (mg) |
Recovery a %± SD b |
Lactose |
50 |
99.6 ± 0.8 |
Glucose |
50 |
98.8 ± 0.6 |
Dextrose |
50 |
99.3 ± 0.7 |
Magnesium stearate |
30 |
99.2 ± 0.7 |
Calcium hydrogen phosphate |
50 |
99.5 ± 0.9 |
Talc |
40 |
99.8 ± 0.6 |
Starch |
50 |
100.0 ± 1.1 |
a 6.0 mg/mL of ETD was taken; b Average of five determinations; SD : Standard deviation. |
||
3.8. Analytical Applications
The proposed methods were successfully applied to determine ETD in its tablets. Therefore,
they could be used easily for the routine analysis of pure ETD and its dosage forms.
Moreover, to check the validity of the proposed methods, dosage form [Napilac capsules
(200 mg ETD per capsule) and Etodine capsules 300 mg ETD per capsule)] were tested for
possible interference with standard addition method. The performance of the proposed
methods was assessed by calculation of the t-test (for accuracy) and a variance ratio
F-value (for precision) compared with the reference method (potentiometric titrate with
tetrabutylammonium hydroxide) (for 95% confidence level with five degrees of freedom. The
results showed that the t- and F-values were less than the critical value, indicated that
there was no significant difference between the proposed and reference method for ETD.
Because the proposed methods were more reproducible with high recoveries than the
reference method, they can be recommended for the routine analysis in the majority of
drugs quality control laboratories.
4. CONCLUSIONS
The proposed method is simpler, less time consuming and more sensitive than the
published method. The proposed method was advantageous over other reported visible
spectrophotometric method with respect to their higher sensitivity, simplicity,
reproducibility, precision, accuracy and stability of the colored species for ³ 12 h.
The proposed method is suitable for the determination of ETD in pure form and in tablets
without interference from excipients such as starch and glucose or from common degradation
products, suggesting applications in bulk drug analysis.
REFERENCES
[1] British Pharmacopiea, (2004), CD-ROM, 4th Ed., Volume 1, Monographs: medicinal and
pharmaceutical substances.
[2] El Kousy N.M., (1999) J. Pharm. Biomed. Anal., 20,185-194.
[3] Duymus H., M. Arslan, M. Kucukislamoglu, M. Zengin, (2006) Spectrochimica Acta Part
A., 65, 1120-1124.
[4] Amer S.M., El-Saharty Y.S., Metwally F.H., Younes K.M., (2006) J. AOAC Int., 88,
1637-1643
分光光度法测定依托度酸的研究
(1昆明理工大学材冶学院,云南昆明650031;2云南民族大学化学与生物技术学院,云南昆明 650031;3 云南大学化学系,云南昆明650091)
摘要 研究了用分光光度法测定依托度酸,在pH 3.5 ~ 6.0的醋酸-醋酸钠缓冲介质中,依托度酸可还原Fe3+为Fe2+,还原生成的Fe2+和2,2’-联吡啶生成红的络合物,其吸光度和依托度酸浓度呈线性关系,在500 nm波长处测定,依托度酸的浓度在0.5-25 mg/mL内符合比尔定律,方法用于依托度酸度酸片剂中依托度酸含量的测定,相对标准偏差小于0.82%,标准回收率在97% - 102%之间,结果令人满意。
关键词 依托度酸;2,2’-联吡啶;分光光度法