http://www.chemistrymag.org/cji/2008/106032pe.htm |
Jun.8, 2008 Vol.10 No.6 P.32 Copyright |
Lu Yunkai, Zhao Ning, Qin Xinying, Liu Yue,
Lu Guodong
(College of Chemistry and Environmental Science, Hebei University, Key Laboratoryo f
Analytical Science and Technology of Hebei Province, Baoding 071002, China)
Abstract A new molecularly imprinted
polymer was synthesised based on sacrificial mesoporous silica (Si-MIP) for the
solid-phase extraction of fluoroquinolone residues in edible animal products. The Si-MIP
was prepared by filling mesoporous silica with the template molecule and monomers followed
by polymerisation, then the silica particles materials were removed from the obtained
imprinted composites. The binding capacity was evaluated by static adsorption and
Scatchard analysis, which showed that the dissociation constant (KD) and
the maximum binding capacity (Qmax) were 178.9 mg L-1 and
73.31 mg g-1 for high affinity binding site, and 45.54 mg L-1
and 40.82 mg g-1 for lower affinity binding site, respectively. The largest
selectivity coefficient for ofloxacin in the presence of enrofloxacin was found to be
3.79, the largest relative selectivity coefficient between ofloxacin and enrofloxacin over
3, along with a fast kinetics comparing with MIP without silica template. The SPE
procedure was optimized, and the accuracy and selectivity of Si-MIP cartridge process
developed were verified using a MIP cartridge and a classical C18 cartridge as the SPE
matrix during control experiments. The quantification and detection limits in tissue
samples of ofloxacin, enrofloxacin and flumequin and were established at 18 m g kg-1,
21 m g kg-1 and 42 m g kg-1, respectively.
Keywords Molecularly imprinted polymers; Solid-phase extraction; quinolones; Silica
gel as sacrificial material; Tissue sample
1. INTRODUCTION
Molecular imprinting technique (MIT) is
an increasingly developing technique for preparing polymers with desired and predetermined
selectivity, and provides specific binding sites or catalytic sites in molecularly
imprinted polymers (MIPs). MIPs had been attracted extensive attention and have been used
extensively in sensors, immunoassay-type binding assays in place of antibodies and the
separation techniques, which involve affinity chromatography, capillary
electrochromatography, solid phase extraction (SPE), thin layer chromatography and
membrane separation[1]. A particularly promising application of MIPs is as
selective sorbents in SPE for the cleanup and preconcentration of compounds from low
concentrations or complex matrices[2, 3]. The key of molecularly imprinted
solid phase extraction (MISPE) development is preparations of the molecular imprinting
sorbents.
The conventional method is to synthesize the MIPs in bulk thermal-(or
photo-)polymerisation that produces a monolithic polymer that has to be grinded and
sieved, resulting in irregularly shaped materials with heterogeneous size and porosity.
Although this technique has led to highly selective materials for a multitude of analytes,
such particles are not well suited as packing materials for SPE. For example, high
backpressures and low mass transfer kinetics are usually observed. Sacrificial materials
can overcome these problems, and these synthesic methods have been published, such as
filling support particles[4].
In this study, we apply a MIPs preparation protocol based on
sacrificial mesoporous silica[4] to prepare a new SPE sorbent with high selectivity and high mass
transfer kinetics for selective separation and preconcentration of the veterinary residue
(e.g. quinolones) from edible animal products.
Quinolones are antibacterial agents widely used in the treatment of
infections in both humans and animal. Their primary target is the bacterial enzyme DNA
gyrase or topoisomerase II, which renders the DNA molecule compact and biologically active[5].
Quinolones are widely used in veterinary medicine, and the problem of veterinary residue
at animality foodstuff become societal focus. The European Union and the Joint FAO/WHO
Expert Committee on Food Additives (JECFA) have established maximum residue limits (MRL)
for several quinolones. The harmful of veterinary residue for people and environment is
chronic, long-dated and cumulate. When prople eat the animality foodstuff which go beyond
standard mete of the veterinary residue, the health or life will be endanger[6].
Quinolone residue analysis involves extraction with an appropriate
solvent followed by one or more clean-up processes and determination by HPLC, LC-MS, CE,
CE-MS, ELISA. The sample treatment is important procedure before quinolone residue
determination in order to reduce the matrix interference and enrich the analytes.
Selective extraction of quinolone drug from foodstuff is usually achieved by solvent
extraction and solid phase extraction (SPE), the latter is more rapid, simple, economical,
environmental friendly and easy automatization than the traditional liquid–liquid extraction (LLE). Increasing the selectivity of sorbent in
the extraction of analytes and developing new efficient cleanup techniques are highly
attractive for monitoring trace analytes in complex samples, So, development of new solid
sorbents for selective separation quinolone residues in food, therefore, is of great
significance[7].
The development of the molecular imprinting technique about quinolone
medicament have some study and put up huge potential and advantage[8-12]. In
this work, A new preparation method of molecularly imprinted polymer based on sacrificial
mesoporous silica (Si-MIP) was investigated, and the quinolone imprinted polymer was used
as SPE sorbent for separation and preconcentration of the quinolone residue from edible
animal products.
2. EXPERIMENTAL
2.1 Chemicals
All the chemicals used were of analytical reagents: Methacrylic acid (MAA) and
ethylene glycol dimethacrylate (EGDMA) from Sigma-Aldrich (Shanghai) Trading Co., Ltd.
(Shanghai, China) and cleaned to remove the inhibitor prior to polymerization.
2,2-Azobisisobutyronitrile (AIBN)from Beijing Chemical Reagent company (Beijing, China)
recrystallized prior to use. Ofloxacin (OFL), enrofloxacin (ENR) and flumequine (FLU) were
obtained from the National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China). The structures of the studied compounds are shown in Fig. 1.
Anhydrous alcohol, acetone, acetonitrile (ACN), chloroform and methanol were all of HPLC
grade and purchased from Tianjing Kermel Chemical Reagents Development Centre (Tianjin,
China). Trichloroacetic acid (TCA) and acetic acid (analytical grade) was purchased from
Jinli Industries Co. (Tianjin, China). All of the solutions were filtered through a 0.45 m
m membrane filter (Millipore) before use. Doubly deionized water (DDW) was used
throughout.
Commercially available silica particles (20 m m mean diameter, 0.7 ml g-1
pore volume, 8.0 nm pore size) were obtained from Qingdao Makll Group (Qingdao, Shandong,
China). Silica gel was first activated by reflux in conc hydrochloric acid for 4 h to
remove any adsorbed metal ions, then filtered, washed repeatedly with doubly distilled
water to neutral filtrate and dried in an oven at 160°C for 8 h to remove surface water.
Stock standard solutions (1 g L-1) of each standard were
prepared in acetonitrile. Standard solutions were prepared daily by diluting stock
solutions in CHCl3 to concentrations of 0.02, 0.05, 0.1, 0.15, 0.2, 0.3, 0.4
and 0.5 g L-1 ofloxacin. All solutions were stored in the dark at -4ºC.
Fig. 1 The chemical structures of ofloxacin,
enrofloxacin and flumequine
2.2 Instrumentation and chromatographic conditions
A Shimadzu (Kyoto, Japan) LC-6A Liquid Chromatographic System with UV detector with a
spectral range from 200 to 600 nm was used to analyze the tested solutions. The
chromatographic analysis were performed on a Kromasil C18 column (250 mm×4.6 mm I.D.,
particle size 5 m m). The mobile phase was 0.025 mM orthophosphoric acid-acetonitrile
(70:30) containing 2.5 mM 1-heptanesulfonic acid and the flow rate was 1 mL min-1
at ambient temperature. Aliquots of 20 m L were injected into the column and the
chromatograms were recorded spectrophotometrically at 284 nm.
2.3 Preparation of MIP
A sacrificial silica imprinting concept [4] was applied to the preparation of a
new molecularly imprinted polymer based on sacrificial mesoporous (Si-MIPs) for selective
solid phase extraction of the quinolone residue from edible animal products.
For pre-polymerisation mixtures preparation, 0.361 g (1 mmol) of
template (ofloxacin), 0.34 ml (4 mmol) of MAA and 3.77 ml of EGDMA (20 mmol) were
dissolved in 14 ml of chloroform in a 25 ml screw-capped glass vial and sonicated. After
complete dissolution, the solution was churn up 30 min and then purged with N2
for 5 min. In order to obtain discrete silica–polymer
particles and to prevent sticky particle agglomerates. Finally the solution was allowed to
stand for 24 h at 40ºC.
The pre-polymerisation mixture was added to 3.000 g silica and
shaken vigorously. The mixture was then gently stirred with a spatula until all the
mixture penetrated the silica pores and distributed uniformly throughout the surface of
silica. Subsequently, 0.0164 g of AIBN was dissolved in 3 ml of chloroform and added to
the mixture. Finally, the mixture was sonicated to remove any entrapped air bubbles. A
free-flowing material was then obtained. It was flushed with N2, sealed and
shaked in a mechanical shaker at 60ºC for 24 hour.
After polymerisation was completed, Si-MIP composite materials were obtained.
The obtained composite materials was crushed, ground and wet-sieved
with acetone. The particle size fraction of 54-74 m m was collected. The resulting
particles were transferred into a screw-capped polypropylene tube. The suspension was then
cooled in a water-ice bath and 30 ml of 40% aqueous HF was added in several portions while
shaking the tube. The suspension was allowed to incubate overnight on a rocking table at
room temperature to completely dissolution of the silica matrix of the composite. After
dissolution of the silica, the suspension was diluted with 100 ml deionised water,
filtered on a glass filter funnel (7 m m pores) and washed extensively with 2 L of
deionised water until neutrality. The particles-free HF were placed in a Soxhlet
extraction apparatus and washed with ethanol/glacial acetic acid (8:2, v/v ) solution
until ofloxacin could no longer be detected at 284 nm in the eluent. Then the particles
were washed with methanol to remove residual acetic acid and dried to constant weight
under vacuum at 60ºC. A
non-molecularly imprinted polymer (NMIP) was prepared in the absence of template and a
molecularly imprinted polymer in the absence of Mesoporous Silica Templates (MIP), and
treated in an identical manner.
2.4 The scanning electron microscopy
A scanning electron microscopy (SEM) was employed for the analysis of the
characteristics of MIP and NMIP. SEM analysis was carried out metal coating at room
temperature by using low vaccum mode.
2.5 MISPE cartridges preparation,washing, and elution procedures
A slurry of 100 mg of the Si-MIP or the control polymer (CTL) in 2 mL of MeOH,
respectively, was packed into 1 mL SPE syringe barrels and capped with fritted
polyethylene disks at the top and at the bottom. Prior to each use, the columns were
washed successively with 10%(v/v) acetic acid/methanol (5 mL), acetonitrile (2 ml) and
chloroform (2 ml). As a control, a blank SPE column was also prepared in the same manner
but with the blank polymer.
A 10 ml sample spiked at 100 mg L-1 with a mixture of
fluoroquinolones OFL, ENR and FUL was prepared in chloroform solvent was passed through at
a flow rate of 1 mL min-1, then the cartridge was washed with 5 ml of acetic
acid in ethanol (1:99). The analytes retained in the cartridge were eluted with 5 mL of
acetic acid in methanol (10:90). The collected aliquots from the washing step were
directly analyzed by HPLC. The collected aliquots from the elution step (1 mL) were dried
under nitrogen and redissolved in 1 mL the mobile phase.
2.6 Pretreatment of the biological sample [13, 14]
Muscle sample were obtained from chicken, and were stored at
-10ºC. A sample of liver and muscle (5.00 g wet mass) was accurately
weighed. In order to investigate the effect of spiking procedure on extraction efficiency,
the fluoroquinolone standard solutions were prepared in acetonitrile. 5 g of tissue was
Spiked with the mixture of quinolone at 100m g kg-1. The ground muscle or liver
was added a mixture containing 10% TCA-acetonitrile (8:2) and then the sample was
homogenized. After homogenization the tube content was centrifuged at 3500 g for 10 min (4ºC). The supernatant was filtered, dried in a gentle stream of
nitrogen, redissolved in 10 ml chloroform solvent and passed through the MISPE cartridge.
The washing, elution, and nanlytical procedures were the same as described above.
3. RESULTS AND DISCUSSION
3.1 Polymer synthesis and recognition mechanism
Silica particles are excellent materials as HPLC
stationary phases. They can be dissolved and removed with appropriate reagents and that
makes them very suitable both as a support and sacrificial material for the method
presented[4]. The novel method for preparing Si-MIP based on the sacrificial
mesoporous silica with high surface area, uniformly shaped particles and uniform pore size
was developed. The synthetic MIPs approach involves a pre-polymerisation mixture
penetrated the pores of the silica and accumulated onto the silica surface by
electrostatic interactions in aprotic solvent (i.e. chloroform), and the interaction of
the template molecule with functional monomers is hydrogen bonds in preparing imprinting
polymers, followed polymerisation in the presence of an initiator. Discrete single
silica-polymer composite materials with the shape of the silica support (Si-MIP
composites) were obtained. In a following step, the silica can be dissolved and removed,
resulring in materials consisting of molecularly imprinted polymer.
3.2 Affinity of the imprinted polymer
In order to investigate the binding
performance of the Si-MIP, saturation experiments and subsequent Scatchard analysis were
carried out[15]. The sized and washed polymer particles (100 mg) were mixed
with a 10 mL chloroform solution of ofloxacin of varied concentration from 50 m mol L-1
to 1.4 mol L-1. The mixture was oscillated in a constant temperature bath at 25ºC for
24 h. The mixture was transferred into a centrifuge tube and centrifuged at 4000 rmp for 5
min. The concentrations of free compounds in the solutions were determined by an
UV-Spectrometer at 284 nm. The absorption quantity (Q ) was calculated by
subtracting the free concentrations (Cfree) from the initial concentrations (C0).
The average data of triplicated independent results were used for the Scatchard analysis.
Binding data can be linearly transformed according to the Scatchard equation Q/C0
= (Qmax-Q)/KD where KD is an equilibrium dissociation
constant and Qmax is an apparent maximum number of binding sites. When Q/ Cfree
is plotted versus Q, KD and Qmax can be estimated from the slope and
the intercept, respectively.
As shown in Fig. 2, the Scatchard plot was not linear, suggesting that
the binding sites in Si-MIP are heterogeneous in respect to the affinity for ofloxacin.
Because there are two distinct sections within the plot which can be regarded as straight
lines, it would be reasonable to assume that the binding sites can be classified into two
distinct groups with specific binding properties. Under this assumption the respective KD
values can be calculated to be 178.9 mg L-1 and 45.54 mg L-1,
and the respective Qmax 73.31 mg g-1 and 40.82 mg g-1
of dry polymer.
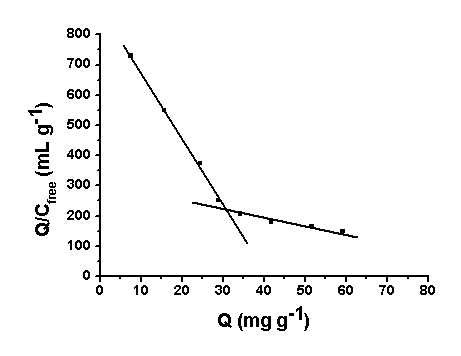
Fig. 2 Scatchard analysis of OFL
3.3 Selectivity of the imprinted sorbent
The structurally similar compound ENR was chosen as the competitive species with OFL for the competitive recognition study. As can be seen in Table 1, distribution coefficient (Kd), selectivity coefficient of the sorbent (k) and the relative selectivity coefficient (k') was obtained in these competitive experiments. Distribution coefficient (Kd) suggested the character of a substance adsorbed by a sorbent, selectivity coefficient of the sorbent (k) suggested the otherness of two substances adsorbed by one sorbent and relative selectivity coefficient (k') suggested the otherness of two sorbents. These factors were calculated as the following formula (1)–(3). OFL and ENR had the similar Kd on the non-imprinted silica sorbent but Si-MIPs showed about four times adsorbed capacity to OFL than to ENR. The k (OFL/ENR) value of the Si-MIP sorbent (3.79) was larger than that of NMIP sorbent (1.13), which showed that the Si-MIP sorbent had high selectivity for OFL over the structurally similar compounds ENR. The k' value was 3.35, which was greater than 1 and showed the Si-MIPs sorbent had higher selectivity than the NMIPs sorbent.
Table 1 Competitive loading of OFL and ENR by Si-MIP and NMIP sorbents
Sorbents |
Initial solutions (mg L-1) |
Final solutions (mg L-1) |
Kd |
k |
k' |
|||
OFL |
ENR |
OFL |
ENR |
OFL |
ENR |
|||
Si-MIP |
500 |
500 |
345 |
447 |
44.93 |
11.86 |
3.79 |
3.35 |
NMIP |
500 |
500 |
482 |
484 |
3.73 |
3.3 |
1.13 |
|
Kd = [(C0 - Ci)/Ci]
× [volume of solution (ml) / mass of gel (g)] (1)
C0 and Ci represent the initial and final
concentrations
k = KdOFL / KdENR (2) k' =kimprinted / knonimprinted (3)
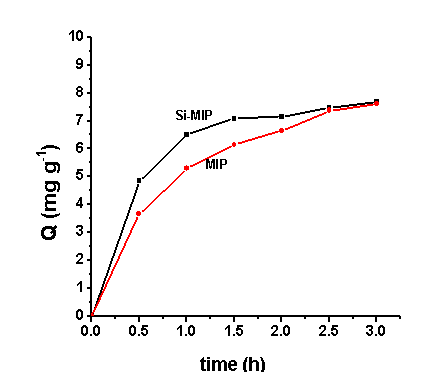
Fig. 3 Dynamics adsorption curve of OFL
MIP: molecular imprinting polymer based on ofloxacin template; Si-MIP: MIP based on
ofloxacin and silica-gel template.
3.4 Adsorption dynamics
In a typical uptake kinetics test, 100 mg of Si-MIP was added to 10 mL of 100 mg L-1 ofloxacin chloroform solution in a 25 ml screw-capped glass vial. The mixture was mechanically shaken for 0.5, 1, 1.5, 2.5, 3 h at room temperature. The mixture was transferred into a centrifuge tube and centrifuged at 4000 rmp for 5 min, then the concentrations of free compounds in the solutions were determined by an UV-Spectrometer at 284 nm Dynamics method was the same as static method except oscillated different times and at the constant concentration (100 mg L-1). The absorption quantity (Q) was caculated by subtracting the free concentration from the initial concentrations.
As shown Fig. 3, the uptake kinetics of ofloxacin. It is clear that the solid extracted process of Si-MIPs is fairly rapid, the 95% uptake of ofloxacin was acieved within 1.5 h. Compared with Si-MIP, the molecularly imprinted polymers in the absence of mesoporous silica templates adsorb ofloxacin slower, attain 90% at least for 2 h.
|
|
Fig.4 SEM of the materials. Si-MIP: based on ofloxacin and silica-gel template; MIP: molecular imprinting polymer based on ofloxacin template.
The sacrificial silica imprinting protocol
improve adsorption dynamics performance of imprinting materials, which was also elucidated
using the scanning electron microscopy and the micrographs of these materials are shown in
Fig. 4. The SEM image of Si-MIP with MIP comparison showed that there were many macropores
and uniform pore size distribution in the network skeleton of imprinted polymers, which
allow the achievement of very high adsorption-desorption efficiencies.
3.5 Optimisation of the MISPE procedure
The optimisation of the whole MISPE procedure (conditioning,
loading and elution) conditions of the steps, a standard solution of OFL was applied to
the determination of the selectivity of the Si-MIP in SPE and achieve good recoveries for
the analyte of interest[10, 11]. The conditoning and the loading steps were
optmised first of all. Thus, 10 mL of sample containing 100 mg L-1 of three
fluoroquinolones (OFL, ENR and FLU) was prepared in chloroform and passed through the
MISPE.
The cartridge had been conditioned previously with 10% (v/v) acetic
acid/methanol (5 ml ), acetonitrile (2 ml) and chloroform (2 ml), and the leakage of MISPE
was checked in each of the MISPE experiments. Chloroform was found to be an optimal sample
solvent for all the analytes were retained on the Si-MIP mainly by selective interactions.
The quinolone molecules have three active sites forming hydrogen bond with Si-MIP for
binding to the Si-MIP during the loading step.
A clean-up step (selective washing step) serve to remove as much as
possible of sample contaminants while analyte and enhance the selectivity of Si-MIP. This
step first optimised when the sample was applied in chloroform. Thus, toluene, water,
methanol ethanol and acetonitrile (ACN) were investigated as potential washing solvents
for the clean-up step. The best results were obtained using 5 ml of acetic acid in ethanol
(1:99). Under these conditions there was an improvement since ENR and FLU were recovered
at a level of 70% and 85%, respectively, whereas OFL remained completely bound to the
Si-MIP. Ethanol/acetic acid was used as the wash solvent in all subsequent experiments.
Under acidic conditions, OFL would be deprotonated for rapid
desorption. Guided by this hypothesis, several acidic solutions were investigated for the
elution. The best conditions found to quantitatively elute and recover all the retained
analytes was to use 5 ml of acetic acid / methanol (10:90).
3.6 Determination of quinolones in spiked chicken samples
The spiked tissue sample was extracted according to section 2.6. Subsequently, the supernatant was filtered, dried in a gentle stream of nitrogen,
redissolved in chloroform or water and passed through the Si-MIP cartridge, MIP cartridge
and C18 cartridge, which previously have been conditioned with 3 ml of methanol followed
by 3 ml of water, and elution was accomplished using 2 ml of HPLC mobile phase. Si-MIP
cartridge and MIP cartridge were performed. The recoveries, reproducility, and LOD of the
tissue extracts were calculated and summarized in Table 2.
Table 2 Recoveries(%), precision, and limits of detection (LOD) of OFL, ENR and FLU after MISPE of Chicken sample (spiked 100 m g/kg)
Compounds |
Si-MIP carrtridge |
MIP carrtridge |
C18 carrtridge |
||||||
Recovery(%) |
RSD |
LOD |
Recovery(%) |
RSD |
LOD |
Recovery(%) |
RSD |
LOD |
|
ofloxacin |
92.5 |
4.2 |
18 |
89.2 |
5.10 |
22 |
63.8 |
3.63 |
19 |
enrofloxacin |
30.4 |
4.7 |
21 |
31.6 |
4.04 |
23 |
58.56 |
4.06 |
12 |
flumequin |
21.7 |
3.2 |
42 |
20.5 |
4.71 |
43 |
72.2 |
3.36 |
16 |
It is clear that C18 cartridges have poorer recoveries for three quinolones compared with Si-MIP cartridges and MIP cartridges. The mechanism of C18 bonded-phase extraction is based on non-polar interactions between the carbon–hydrogen bonds of the sorbent and the carbon–hydrogen of bonds of the analyte[13-15]. This confirmed the reliability and efficacy of the proposed method for the analysis of quinolone residues in real samples.
4. CONCLUSION
In this study, we tested a new
technique to synthesize a ofloxacin molecularly imprinted polymer base on silica-gel
sacrificial material. As the templates, ofloxacin was used here and binding to the
molecularly imprinted polymer was confirmed to be highly selective. This ofloxacin
recognition depend on shape of the pores created on polymer.
The approach, therefor, may have great potential application for
generation of separate materials for chromatogram and solid phase extraction
stationary phase. Using the MIPs as selective solid phase extraction stationary
phase, we analyzed the chicken had not found the ofloxacin and enrofloxacin in that
samples. The study results presented here have substantiated the significant research
interest in molecularly imprinted polymer base on silica-gel sacrificial material due to
their ease of preparation, high separation efficiency, and rapid mass transport.
Acknowledgement This research was supported by National Science Foundation of China (No. 20675024), Natural Science Foundation of Hebei Educational Committee (No. 2006407), China Postdoctoral Science Foundation (No. 2005037629) and Science Foundation of Hebei University.
REFERENCES
[1] Burow M, Minoura N., Biochem. Biophys. Res. Commun., 1996, 227: 419-22.
[2] Caro E., Marce R.M., Borrull F. et al. Trends Anal. Chem., 2006, 25: 143-154.
[3] Baggiani, C., Anfossi L., Giovannoli C. Anal. Chim. Acta, 2007, 591(1): 29-39.
[4] Yilmaz E., Ramstrom O., Moller P., et al. J. Mater. Chem., 2002, 12: 1577-1581.
[5] Hernandez M., Borrull F., Calull M. Trends Anal. Chem., 2003, 22: 416-427.
[6] Hernandez-Arteseros J. A., Barbosa J., Compano R., et al. J. Chromatogr.
A, 2002, 945, 1-24.
[7] Andreu
V., Blasco C., Picó Y. Trends Anal. Chem., 2007, 26: 534-556.
[8] Sun H., Dong X., Lu X. et al.Chinese J. Chromatogr., 2003, 21(3): 233-238.
[9] Du X., Peng T., Li J. Chinese J. Anal. Chem., 2003, 31(6): 720-722.
[10] Caro E., Marce R. M., Cormack P. A.G. et al. Anal. Chim. Acta, 2006, 562: 145-151.
[11] Caro E., Marce R. M., Cormack P. A.G. et al. J. Sep. Sci. 2006, 29: 1230-1236.
[12] Gao J., Liu Z., Liu P., et al. Chemical Journal on Internet, 2007, 9(2): 6.
[13] Posyniak A., Zmudzki J., Semeniuk S. J. Chromatogr. A, 2001, 914: 89-94.
[14] Jing X., Tian W., Zhao C et al. Talanta, 2007, 72: 119-125.
[15] Zhu X., Yang J., Su Q et al. J. Chromatogr. A, 2005, 1092: 161-169.
牺牲介孔硅胶法合成分子印迹聚合物及其在固相萃取鸡肉中氧氟沙星残留的应用研究
吕运开,赵宁,秦新英,刘越,陆国栋
(河北大学化学与环境科学学院,河北省分析科学与技术重点实验室,保定 071002)
摘要 基于介孔硅胶模板和氧氟沙星模板的分子印迹聚合物(Si-MIP)制备方法研究,发展了牺牲硅胶印迹方法和分子印迹固相萃取方法,以及固相萃取-HPLC分离检测动物源食品中的氟喹诺酮类兽药残留的新方法。Si-MIP以硅胶材料为载体,将模板分子和功能单体吸附在硅胶表面和空穴内表面,然后进行聚合,除去硅胶模板和氧氟沙星模板分子,得到分子印迹聚合物。通过静态吸附,考察了印迹聚合物的吸附能力;通过Scatchard分析,分别计算了聚合物高结合位点的离解常数KD (178.9 mg/L)和饱和吸附容量Qmax (73.31 mg/g)及低结合位点的离解常数KD (45.54 mg/L)和饱和吸附容量Qmax (40.82 mg/g)。在恩诺沙星存在的条件下,对氧氟沙星的选择性系数为3.79,氧氟沙星和恩诺沙星的相对选择性系数大于3,同未加硅胶模板的MIP相比,Si-MIP有较好的动力学性质。将固相萃取条件进行了优化,通过与MIP萃取柱和传统的C18萃取柱的比较,结果表明Si-MIP萃取柱在本实验条件下有较好的精确性和选择性,并应用于固相萃取-HPLC分离检测了鸡肉组织中的氧氟沙星、恩诺沙星和氟甲喹,检出限分别为18 m g kg-1,21 m g kg-1 和42 m g kg-1。
关键词 分子印迹聚合物 固相萃取 喹诺酮 牺牲硅胶材料 组织样品
 Si-MIP
Si-MIP MIP
MIP