http://www.chemistrymag.org/cji/2008/107035ne.htm |
Jul.
1, 2008 Vol.10 No.7 P.34 Copyright |
Synthesis and crystal structure of a new binuclear copper(II) complex with benzoic acid
Liu Han-fu1, Zhang Xiu-qing2,
Bai Xian-qun1, Chen Su-yi1, Shen Xue-song1
(1College of Pharmacy, Guilin Medical University, Guilin 541004, China; 2College
of Chemistry, Nankai University, Tianjin 300071, China)
Keywords benzoic acid, crystal structures, binuclear copper complex.
1. INTRODUCTION
Many dimeric copper(II) carboxylates have been
studied because they are of special chemical and biological interest [1-3].
Their preparation, structural, magnetic and spectral properties have frequently been
described [4-6]. It is well-known that carboxylate ligands play an important
role in coordination chemistry. They have many coordination modes. Such as terminal
monodentate, bridging bidentate in a syn-syn, syn-anti, and anti-anti
configuration to two metal centers, chelating to one metal center, and bridging tridentate
to two metal centers [7-10]. Here, we selected Benzoic
Acid as the ligand and obtained a new complex. In this paper, Cu2(L)4(CH3CH2OH)2
was synthesized and characterized by IR, elemental analysis, and X-ray diffraction.
2. EXPERIMENTAL
2.1 Instruments and chemicals
IR spectrum was recorded on a Perkin-Elmer Spectrum one FT-IR Spectrometer in the 4000
- 400 cm-1 region by using KBr pellets. Elemental analysis for C, H and N atoms
were carried out on a Model 2400 II Perkin-Elmer elemental analyzer. All reagents were of
analytical grade from commercial sources and were used without any further purification.
2.2 Synthesis of Cu2(L)4(CH3CH2OH)2
1
BA (0.1221 g, 1 mmol) was dissolved in ethanol (10 mL) and KOH (0.0561 g, 1 mmol) was
added. Then the solution of CuCl2·2H2O (0.0852 g, 0.5 mmol)
in distilled water (5 mL) was added. The mixture was stirred at 80°C for 4 h and then
filtered. The filtrate was allowed to stand in air at room temperature for several days,
yielding blue single crystals suitable for analysis.
Selected IR data (n , cm-1, KBr pellet): 3440.02(vs),
1611.70(s), 1571.12(ms), 1407.07(vs), 1102.92(w), 1071.43(w), 720.18(m), 688.29(w).
Anal. calcd for C32H32Cu2O10:
C, 54.62; H, 4.58; N, 0 %. found: C, 53.50; H, 4.67; N, 0 %.
2.3 X-ray crystallography of 1
Table 1 Crystal data and structure refinement for complex 1
Empirical formula |
C32H32Cu2O10 |
Absorption coefficient (mm-1) |
1.380 |
Formula weight |
703.66 |
Crystal size (mm3) |
0.26 x 0.15 x 0.11 |
Crystal system |
Monoclinic |
q (°) |
1.85 to 25.01 |
space group |
C2/c |
Limiting indices |
-26 ≤ h ≤ 56, -7 ≤ k ≤ 7, -26 ≤ l ≤ 25 |
a (nm) |
4.7458(3) |
Reflections collected |
15163 |
b (nm) |
0.6679(2) |
Independent reflections |
5636 |
c (nm) |
2.2062(3) |
Data / restraints / parameters | 5636 / 0 / 408 |
| b (°) | 113.315(2) | Goodness-of-fit on F2 |
1.030 |
Volume (nm3 ) |
6.4209(13) | R [I > 2 s(I)] |
0.0461 |
Z |
8 |
wR2 |
0.0681 |
Dc (g/cm3) |
1.456 |
Maximum diff. peak (e/nm3) |
0.478 ×10-3 |
F(000) |
2896 |
Minimum diff. peak (e/nm3) |
-0.432 ×10-3 |
A single crystal of 1 with dimensions of 0.26 mm ×
0.15 mm × 0.11 mm was selected and mounted on a Bruker Smart 1000 diffractometer with Mo
Ka radiation (l= 0.071073 nm) by using an w-scan technique at
298(2) K. 15163 reflections were collected in the rang of 1.85 to 25.01, of which 5636 (Rint
= 0.0700) unique reflections and 2309 observed ones with I > 2s (I) were used in the succeeding refinements. The coordinates of the
hydrogen atoms were obtained from difference Fourier maps and refined with a common
isotropic thermal parameter. All calculations were carried out using SHELXS-97[11] and
refined by full-matrix least-squares, based on F2, using SHELXL-97[12]
programs. Details of data collection and processing are given in Table 1.
2 Results and Discussion
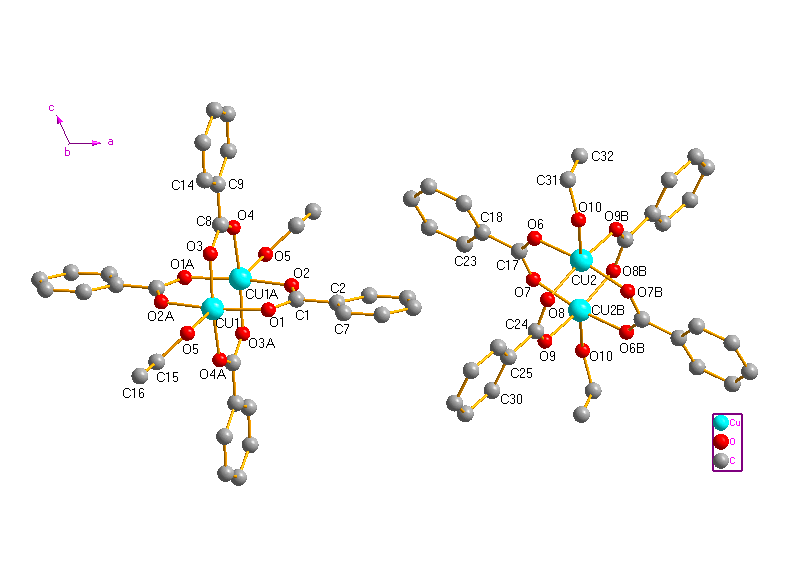
Fig. 1 The
molecular structure of Cu2(L)4(CH3CH2OH)2
(H atoms are omitted for clarity).
The crystal structure consists of two types of symmetrically independent centrosymmetric dimetallic units. Perspective views of the molecules are given in Fig. 1. Selected bond lengths and bond angles are listed in Table 2. In the two units of complex 1, every BA anion adopts bidentate bridging coordination mode using two oxygen atoms of the carboxylate group. The Cu···Cu separations are 0.25893(10) nm (between Cu1and Cu1A) and 0.25990(11) nm (between Cu2 and Cu2B), respectively. Which are comparable well to that in the reported dinuclear caboxylate-bridged complexes [13-17]. Each Cu(II) ion is coordinated by five oxygen atoms from four bridging ligands and one ethanol molecule. The Reejijk distortion index t value [18] is 0.00650 for Cu1 and 0.00016 for Cu2, respectively, indicating slightly distorted square pyramidal (sp) configuration for the copper(II) ions. The equatorial plane of each copper(II) contains of four oxygen atoms from four ligands with the average Cu-O bond lengths of 0.19555 and 0.19562 nm for Cu1 and Cu2, respectively. Cu1 and Cu2 are displaced by 0.07552 and 0.01974 nm out of the basal plane towards the axial ethanol ligand, respectively. The O atom of ethanol molecule occupies the apical position of the coordination sphere. The crystal packing is stabilized by intermolecular O-H···O hydrogen bonds (Table 3 and Fig. 2).
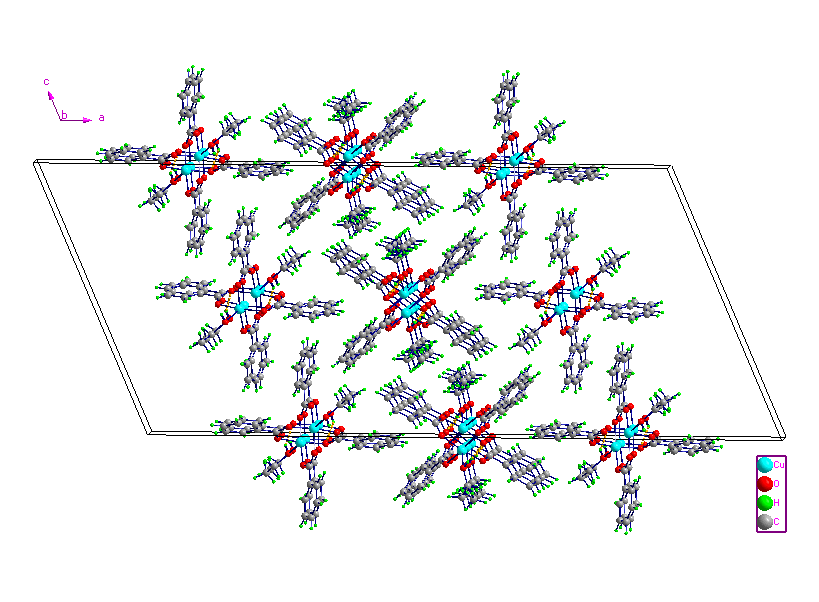
Fig. 2 The crystal packing of 1, showing the O-H···O
hydrogen-bonding interactions as yellow dashed lines.
Table 2 Selected bond lengths (nm) and bond angles (o)
| Bond length | |||
| Cu(1)-O(3) | 0.1947(3) | Cu(2)-O(9B) | 0.1945(3) |
| Cu(1)-O(2A) | 0.1951(3) | Cu(2)-O(6) | 0.1949(3) |
| Cu(1)-O(4A) | 0.1961(3) | Cu(2)-O(8) | 0.1957(3) |
| Cu(1)-O(1) | 0.1963(3) | Cu(2)-O(7B) | 0.1974(3) |
| Cu(1)-O(5) | 0.2157(3) | Cu(2)-O(10) | 0.2142(3) |
| Bond angle | |||
| O(3)-Cu(1)-O(2A) | 89.12(14) | O(9A)-Cu(2)-O(6) | 91.15(15) |
| O(3)-Cu(1)-O(4A) | 169.15(12) | O(9A)-Cu(2)-O(8) | 168.31(13) |
| O(2A)-Cu(1)-O(4A) | 90.57(15) | O(6)-Cu(2)-O(8) | 89.27(14) |
| O(3)-Cu(1)-O(1) | 88.38(14) | O(9A)-Cu(2)-O(7A) | 89.14(15) |
| O(2A)-Cu(1)-O(1) | 168.76(12) | O(6)-Cu(2)-O(7A) | 168.32(12) |
| O(4A)-Cu(1)-O(1) | 89.83(15) | O(8)-Cu(2)-O(7A) | 88.11(14) |
| O(3)-Cu(1)-O(5) | 95.56(11) | O(9A)-Cu(2)-O(10) | 98.23(12) |
| O(2A)-Cu(1)-O(5) | 93.60(12) | O(6)-Cu(2)-O(10) | 93.83(12) |
| O(4A)-Cu(1)-O(5) | 95.28(12) | O(8)-Cu(2)-O(10) | 93.40(12) |
| O(1)-Cu(1)-O(5) | 97.55(12) | O(7A)-Cu(2)-O(10) | 97.68(13) |
Table 3 Hydrogen-bonding geometry (nm, °) for the title complex
D--H···A |
d(D-H) |
d(H···A) |
d(D···A) |
∠DHA |
Symmetry Code |
O5--H5···O1 |
0.0820 |
0.2391 |
0.3124 |
149.41 |
-x+1/2, -y+1/2, -z+1 |
O10--H10···O7 |
0.0820 |
0.2272 |
0.3091 |
177.05 |
x, y-1, z |
REFERENCES
[1] Kawata T, Uekusa H, Ohba S. Acta Cryst., B48, 1992: 253.
[2] Yang R N, Xue B Y, Wang D M et al. Chin. J. Chem., 1997, 15: 76.
[3] Del Sesto R E, Arif A M, Miller J S. Inorg. Chem., 2000, 39: 4894.
[4] Melnik M. Coord. Chem. Rev., 1982, 42: 259.
[5] Valko M, Bilton R F, Morris H et al. J. Coord. Chem., 1993, 29: 257.
[6] Sundberg M R, Uggla R, Melnik M. Polyhedron, 1996, 15: 1157.
[7] Rueff J M, Masciocchi N, Rabu P et al. Eur. J. Inorg. Chem., 2001: 2843.
[8] Policar C, Lambert F, Cesario M et al. Eur. J. Inorg. Chem., 1999: 2201.
[9] Ribot F, Toledano P, Sanchez C. Inorg. Chim. Acta, 1991, 185: 239.
[10] Yin M C, Yuan L J, Ai C C et al. Polyhedron, 2004, 23: 529.
[11] Sheldrick G M SHELXS-97, Program for X-ray Crystal Structure Solution [M]. University
of G?ttingen : Germany, 1997.
[12] Sheldrick G M SHELXL-97, Program for the Refinement of Crystal Structures [M].
University of G?ttingen: Germany, 1997
[13] Wu B, Wang G C. Acta Cryst. E, 2004, 60: m1764.
[14] Wang Y Y, Shi Q, Shi Q Z et al. Polyhedron, 2000, 19: 891.
[15] Benisvy L, Quesada M, Turpeinen U et al. Chem. Commun, 2006: 2373.
[16] Calvo P V, Vega A, Spodine E. Organometallics, 2006, 25: 1953.
[17] Deka K, Barooah N, Sarma R J et al. J. Mol. Struct., 2007: 44.
[18] Addison A W, Rao T N, Reedijk J et al. J. Chem. Soc. Dalton Trans., 1984: 1349.
刘汉甫1*,张秀清2,白先群1,陈素一1,沈雪松1
(1桂林医学院药学院,桂林,541004; 2南开大学化学学院,天津,300071)
摘要 本文合成了一个新的双核铜配合物Cu2(L)4(CH3CH2OH)2(HL=苯甲酸),并通过红外、元素分析、X射线衍射等方法对其结构进行表征。该单晶属单斜晶系,空间群为C2/c,晶体结构表明,每两个Cu2+通过四个配体桥联形成一个双核配合物中。每个Cu2+处于一个畸变的四方锥配位环境中,配位原子分别来自四个配体羧基上的氧原子以及一个乙醇氧原子。
关键词 苯甲酸,晶体结构,双核铜配合物。