http://www.chemistrymag.org/cji/2008/107036pe.htm |
Jul.
1, 2008 Vol.10 No.7 P.36 Copyright |
Kinetics and mechanism of oxidation of 1,3-butylene glycol by diperiodatocuprate (III) complex in alkaline medium
Wang
Liping, Ge Xusheng, Wang Yaru, Xu Mingyuan
(Department of Chemistry, Baoding University, Baoding 071000, China)
Abstract The kinetics of oxidation of
1,3-butylene glycol by diperiodatocuprater(III)(DPC)in alkaline medium have been studied
by spectrophotometry in the range of 298.2-313.2K. It is shown that the reaction was first
order with respect to each reductant and Cu (III) , and reaction rates decrease with
increase in [IO4-] and increase in [OH-]. A plausible
mechanism of reaction involving a pre-equilibrium between DPC and [Cu(OH)4]-
was proposed, which could be applied to explain all experimental phenomena, and the
activation parameters of the rate-determining step have been also calculated.
Keywords diperiodatocuprater(III), 1,3-butylene glycol, redox reaction, kinetics
and mechanism
Transition metals in a higher oxidation state can generally be stabilized by chelation with suitable polydentate ligands, while metal chelates such as diperiodatocuprate(III)[1], ditelluratocuprater(III)[2], diperiodatonickelate(IV)[3] are good oxidants in a medium with an appropriate pH value. The use of Cu(III) as an oxidation agent in analytical chemistry has been reported[4], but most of these are o-compounds, Therefore, it was thought worthwhile to study the kinetics of oxidation of some m-compounds such as 1,3-butylene glycol, and investigation will certainly provide us with more dynamical parameters theoretical foundation for the design of reaction route in the organic synthesis and quantitative analysis in analytical chemistry. In this paper, the mechanism of oxidation of 1,3-butylene glycol by diperiodatocuprater(III) is reported.
1. EXPERIMENTAL1.1 Materials
All reagents used were of AR grade. All solutions were prepared with doubly distilled water. Solutions of DPC and reductant were always freshly prepared before use. The stock solution of DPC in a strong alkaline medium was prepared by the method given by Jaiswal[4]. The ionic strength was maintained by adding KNO3 solution and the pH value was regulated with KOH solution.
1.2 Kinetic measurement and reaction product analysis
Measurements of the kinetics were performed using a UV-1901 spectrophotometer (Beijing) fitted with a CS-501 thermostat (±0.1ºC, Chongqing).The kinetics measurements were described previously[5]. The product of oxidation was the corresponding aldehyde alcohols by their characteristic spot test[6]. 2. RESULTS AND DISCUSSION
2.1 Evaluation of pseudo-first order rate constants
The calculated measurements of the rate constant (kobs) values were described previously[5]
2.2 Effect of varying [1,3-butylene glycol]
At fixed [Cu(III)], [OH-], [IO4-], ionic strength and temperature, k obs values increased with increase in [1,3-butylene glycol] and the plots of kobs versus [1,3-butylene glycol] were line at passing through the origin (Fig.1) indicating that the reaction is first order in reductant.
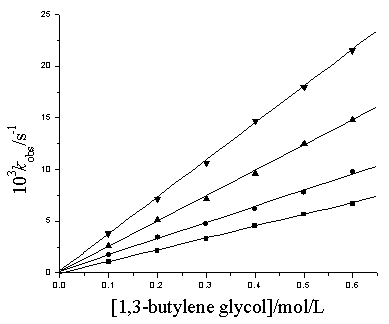
Fig.1 Plots of kobs vs [1,3-butylene glycol] at different temperatures
[Cu(III)]=8.00×10-5mol/L, [IO4-]=1.60×10-3mol/L, [OH-]=0.10mol/L, m=0.10mol/L
2.3 Effect of varying [OH-]
At fixed [Cu(III)],[1,3-butylene glycol], [IO4-],
ionic strength and temperature, kobs values increased with increase in
[OH-], the plots of lnkobs versus ln[OH-] were
linear(r≥0.996) and
from the slopes of such plots the observed order, nap, were found to be 1<nap<2.
In addition, the plots of 1/kobs versus f2 ([OH])/[OH-]2
were linear (Table1 ).
Table 1 103 kobs
/s-1 varying with the different [OH-] at different temperatures
[Cu(III)]=8.00×10-5mol/L,
[IO4-]=1.60×10-3mol/L, [1,3-butylene glycol]=0.20 mol/L,
m=0.25
mol/L
[OH-]/mol/L |
0.05 |
0.08 |
0.12 |
0.16 |
0.20 |
0.25 |
b |
r |
T/K |
||||||||
298.2 |
0.899 |
1.646 |
2.745 |
3.914 |
5.415 |
6.358 |
1.24 |
0.999 |
303.2 |
1.252 |
2.338 |
3.897 |
5.700 |
8.123 |
9.466 |
1.29 |
0.998 |
308.2 |
2.025 |
3.868 |
6.209 |
9.745 |
13.08 |
15.34 |
1.29 |
0.998 |
313.2 |
2.602 |
5.113 |
8.862 |
13.60 |
17.69 |
20.33 |
1.32 |
0.996 |
b and r respectively stand for the slope and the relative coefficient of the plot of lnkobs vs lnC
2.4 Effect of varying [IO4-]
and ionic strength m
At fixed [Cu(III)], [OH-],
[1,3-butylene glycol], ionic strength and temperature, kobs decreased
with increase in [IO4-]. The observed orders, nap, were
found to be -1.36. The plots of 1/kobs versus [IO4-]2
was linear (Table 2). Table 2 reveals that ionic strength has negligible effect on the
rate.
Table 2 Influence of variation of [IO4-], and ionic strength m, t=303.2K
105[Cu(III)]/mol/L |
[1,3-butylene glycol]/mol/L |
103[IO4-]/mol/L |
[OH-]/mol/L |
m/mol/L | 103 kobs/s-1 |
8.00 |
0.40 |
0.80 |
0.10 |
0.10 |
17.65 |
8.00 |
0.40 |
1.60 |
0.10 |
0.10 |
6.838 |
8.00 |
0.40 |
2.40 |
0.10 |
0.10 |
4.548 |
8.00 |
0.40 |
3.20 |
0.10 |
0.10 |
2.726 |
8.00 |
0.40 |
4.00 |
0.10 |
0.10 |
1.897 |
8.00 |
0.30 |
1.60 |
0.08 |
0.08 |
3.738 |
8.00 |
0.30 |
1.60 |
0.08 |
0.16 |
3.454 |
8.00 |
0.30 |
1.60 |
0.08 |
0.24 |
3.550 |
8.00 |
0.30 |
1.60 |
0.08 |
0.32 |
3.534 |
8.00 |
0.30 |
1.60 |
0.08 |
0.40 |
3.733 |
2.5 Free radicai detection
Acrylamide was added under nitrogen blanket
during the course of reaction. The appearance of white polyacrylamide was consistent with
free radical intermediates in the oxidation by Cu(III) complexes. Blank experiments gave
no polymeric suspensions.
2.6 Discussion
In aqueous periodate solution equilibria
(1)~(3) were detected and the corresponding equilibrium constants at 298.2K were
determined by Aveston[7].
![]() 2 IO4- + 2 OH-
H2I2O104- log b1=15.05
(1)
2 IO4- + 2 OH-
H2I2O104- log b1=15.05
(1)
![]() IO4-+ OH-+H2O
H3IO62- log b2=6.21
(2)
IO4-+ OH-+H2O
H3IO62- log b2=6.21
(2)
![]() IO4- + 2 OH-
H2IO63- logβ3=8.67
(3)
IO4- + 2 OH-
H2IO63- logβ3=8.67
(3)
The distribution of all species of periodate in aqueous alkaline
solution can be calculated from equilibria (1)-(3). Under the conditions of [OH-]=0.01~0.1mol·L-1,
[H2IO63-]: [H3IO62-]: [H2I2O104-]:
[IO4-]≈(2.9~29): 1.0:
(0.02~0.2): (6×10-5~6×10-2) , the dimer and IO4- species
of periodate can be neglected, Neglecting the concentration of ligand dissociated from Cu
(III) and the species of periodate other than H2IO63- and
H3IO62-, Eqs. (4) and (5) can be obtained from(2) and(3):
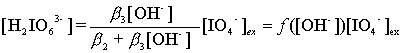 (4)
(4)
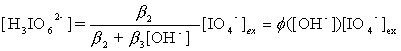 (5)
(5)
Here [IO4-]ex represents the original
overall entering periodate and equals approximately to the sum of [ H2IO63-
] and [H3IO62-].
Because the hexa-cyclic compound formed by m-compound is larger, will
be more spatial hindrance compared to penta-cyclic compound formed by o-compound, and
[Cu(OH)4]-, being small gives less sterical hindrance than [Cu(OH)2(H2IO6)]2-.
The plot of 1/kobs versus f2 ([OH])/[OH-]2
is linear and the plot of 1/kobs versus [IO4-]2
is also linear with a positive intercept indicating that OH- takes part in a
pre-equilibrium with Cu(III) before the rate-determining step and a dissociation
equilibrium in which the [Cu(III)] loses two periodate ligand H2IO63-
forming [Cu(OH)4]- as reactive species.
In view of the above discussion, a plausible reaction mechanism was
proposed:
[Cu(OH)2(H2IO6)2]5-
+ 2OH- ![]() [Cu(OH)4]-
+ 2H2IO63- (6)
[Cu(OH)4]-
+ 2H2IO63- (6)
[Cu(OH)4]-+ H2CCHOHCH2CH2OH
![]() Cu(II) + H2CCHOHCH2·CHOH
(7)
Cu(II) + H2CCHOHCH2·CHOH
(7)
Cu*(III) +OH- + H2CCHOHCH2·CHOH![]() Cu(II) + H2CCHOHCH2CHO
(8)
Cu(II) + H2CCHOHCH2CHO
(8)
Where Cu*(III) stand for any kind of form which Cu(III) existed in
equilibrium. Subscripts T and e stand for total concentration and at equilibrium
respectively. [Cu(III)]T =[Cu(OH)2(H2IO6)2]e5-
+[Cu(OH)4]-e. Reaction (8) is the rate-determining step.
As the rate of the disappearance of Cu (III) was monitored, the rate of
the reaction can be derived as:
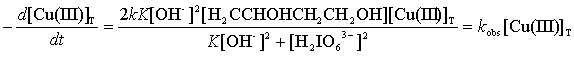 (9)
(9)
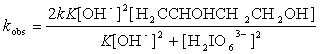 (10)
(10)
Substituting Eq (4) into (10), we get the following equation (11)
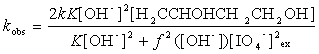 (11)
(11)
Rearranging Eq (11) leads to Eqs (12) and (13):
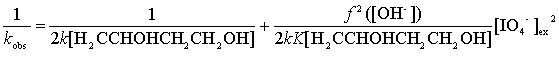 (12)
(12)
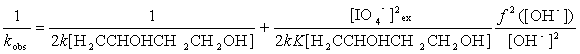 (13)
(13)
Equation (12) suggests that the plot of 1/kobs versus
[IO4-]2ex should be linear, and Equation (13)
shows that the plots of 1/kobs versus f2([OH-])/[OH-]2
should also be linear at different temperatures. From their slopes and intercepts the
rate-determining step constants (k) and equilibrium constants (K) were
evaluated. The activation parameters data of 1,3-butylene glycol obtained by the method
given earlier [8](Table 3).
Table 3 Rate constants (k) and activation parameters of the rate-determining step, equilibrium constants (K)
102k /L/mol· s |
T /K |
Activation parameters (298.2K) |
|||||
298.2 |
303.2 |
308.2 |
313.2 |
Ea |
DH≠ |
DS≠ J/K·mol |
|
1,3-butylene glycol |
1.705 |
2.587 |
4.306 |
6.420 |
69.67 |
67.19 |
-53.55 |
1,3-propyleneglycol see[1] |
2.595 |
4.100 |
6.302 |
8.954 |
64.41 |
61.93 |
-67.44 |
104 K |
|||||||
1,3-butylene glycol |
1.325 |
1.203 |
1.170 |
0.997 |
|||
1,3-propylene glycol see[1] |
1.161 |
0.976 |
0.934 |
0.921 |
|||
The plots of lnk
vs 1/T have following intercept (24.02) slope (-8379.95) and relative coefficient (0.999)
If the formula of DPC was [Cu(OH)2(H3IO6)2]3-,
Equation (14) would be obtained instead of Equation (13).
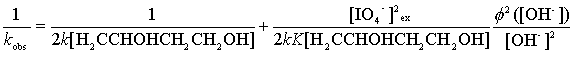 (14)
(14)
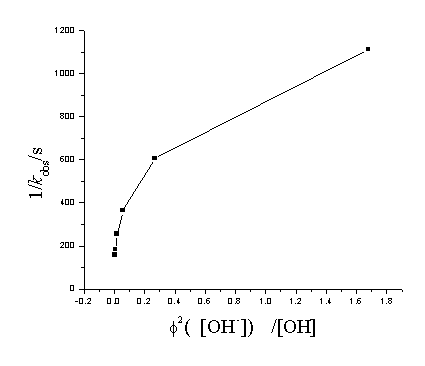
Fig2 Plot of 1/kobs vs
[Cu(III)]=8.00×10-5mol/L, [IO4-]=1.60×10-3mol/L, [1,3-butylene glycol]=0.20mol/L, m=0.25mol/L The plot of 1/kobs versus f2 ([OH-])/[OH-]2 is shown in Fig2 which rules out Equation (14). Based on the experimental observations, the formula of Cu(III) periodate complex may be represented by [Cu(OH)2(H2IO6)2]5-, which is support from kinetic studies.
Comparition 1,3-butylene glycol with 1,3-propyleneglycol (Table 3), we can say that smaller the carbon chain is, the larger the observed rate constants and the rate-determining step constants are, which is consistent with dihydric alcohol's spatial hindrance.
REFERENCES
[1] Wang L P. Shan J H. Xu M Y et al.
Journal of Hebei University, 2006, 26(6): 602.
[2] Shan J H. Wang L P. Huo S Y et al. Chemical Journal on Internet, 2002, 4(10):
49.
[3] Shan J H. Qian Jing. Wang L P et al. Chemical Journal on Internet, 2003, 5(3):
21.
[4] Jaiswal P K. Yadava K L. Indian J Chem, 1973,11: 837.
[5] Shan J H. Wang L P. Shen S G et al. Turkish Journal of Chemistry, 2003, 27: 265.
[6] Feigl F. Spot Tests in Organic Analysis. Elsevier Publishing Co.:
New York, 1956 .
[7] Aveston J. J Chem Soc(A), 1969,273.
[8] Shan J H. Liu T Y. Acta Chim Sinica, 1994, 52: 1140.
王立平 葛旭升 王亚茹 许明远
(保定学院化学系,河北 保定 071051)
摘要 在碱性介质中,用分光光度法研究了二过碘酸合铜(Ⅲ)配离子(DPC) 氧化1,3-丁二醇的反应动力学及机理。结果表明,反应对DPC和1,3-丁二醇均为一级,表观速率常数kobs随着[IO4-] 增加而减小,随着[OH-]增大而增大。据此提出了包括DPC和[Cu(OH)4]-形成的前期快速平衡的反应机理,由假设反应机理推出的速率方程能解释全部实验现象,进一步求得速控步的速率常数k和平衡常数K及活化参数。
关键词 二过碘酸合铜(Ⅲ);1,3-丁二醇; 氧化还原反应; 动力学及机理