http://www.chemistrymag.org/cji/2008/108041ne.htm |
Aug.
1, 2008 Vol.10 No.8 P.41 Copyright |
A new approach to synthesize oxalic acid from formic acid by contact glow discharge plasma
Gao
Jinzhang, Fu Yan, Wang Aixiang, Wu Jianlin, Li Yan, Yang Wu
(College of Chemistry & Chemical Engineering,Northwest Normal University, Lanzhou
730070, China)
Received June 6, 2008.
Abstract A new approach to synthesize oxalic acid from formic acid by using contact glow discharge plasma was made successfully. The product obtained was characterized by using high-performance liquid chromatography, FT-IR spectra, elemental analysis and melting point apparatus. The effects of the ratio of reactant, reaction time and temperature were examined in detail. The yield of oxalic acid (after recrystallizing) can reached 10.6% under the following conditions: the ratio of 5:95 (v/v) for formic acid to water, the reaction time of 90 min, and the reaction temperature of 30ºC.Keywords synthesis, oxalic acid, contact glow discharge plasma.
1. INTRODUCTION
Contact glow discharge is a kind of non-thermal plasma which creates a lot of energetic
species such as ·OH, ·H, e-aq, HO2·,
H2O2, and so on; and these energetic species can be used to induce
some unusual chemical changes in the solution
H2Ogas →H2O+gas + e-aq
H2Ogas →·OH + ·H
H2O+gas + H2O →·OH + H3O+
·OH + HCOOH →·COOH + H2O
·COOH +·COOH →HOOC-COOH
The above results imply that a potential application in the synthetic chemistry would appear in future.
The primary aim of this study is to obtain the solid oxalic acid. It is not a simple way to do so, because the attack coming from the energetic species to substances in aqueous solution is no selection. In other words, the oxalic acid formed could also be destroyed by energetic species. Thus, variable parameters should be optimized firstly in this study.
2. EXPERIMENTAL
The experimental apparatus used consists of a high voltage power supply and a reactor just
as shown in previous works
General procedure for synthesizing oxalic acid was given below: a desired ratio of formic acid to distilled water was added into a 100 mL flask with stirring, the reaction with glow discharge was carried out at a certain temperature and time. After finishing the reaction, the resulting solution was evaporated to near dryness and then, brought to room temperature, finally, filtered to obtain the white crystal. Recrystallization was done with distilled water. Elemental analysis data were calculated for a formula of C2H2O4·2H2O (Found): C,19.05 (19.23); H,4.76 (5.52); O,76.19 ( 75.25). After dehydrating, the melting point of product was detected by WRS-1B digital melting (without correction) to be 186.5-187.9ºC, implying that a good quality oxalic acid was prepared. To assess further the quality of product, the FT-IR spectra of two oxalic acid samples, in which one is analytical reagent grade, were given in Figure 1.
It is clear that two profiles are the same. The broad absorption near 3430 cm-1 was devoted by the stretching vibration of -OH group; the peak at 1690 cm-1 was attributed to the C=O group; the absorption at 1257 cm-1 was corresponding to the C-O stretching vibration; and the absorption at 722 cm-1 should be the structure vibration of C-C group.
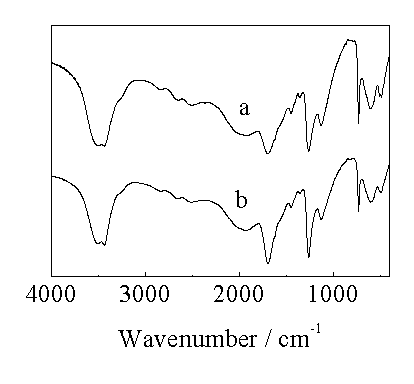
Figure 1. FT-IR spectra of oxalic acid
a: synthesized product; b: analytical reagent
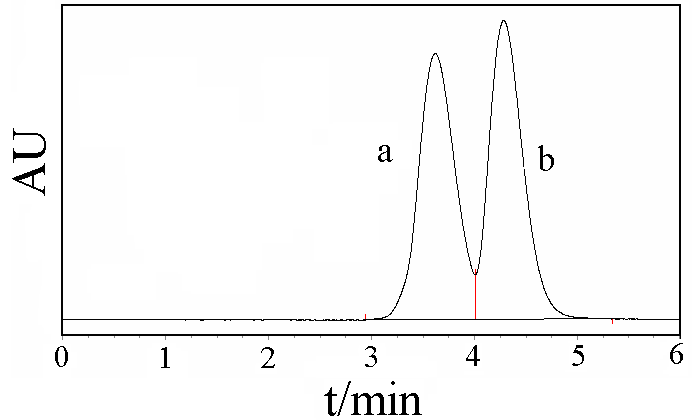
Figure 2. Chromatograms of formic acid under glow discharge plasma
a: formic acid; b: oxalic acid
3. RESULTS AND DISCUSSION
Roughly speaking, the yield of oxalic acid is proportional to the amount of formic acid in
solution, as listing in Table 1. Note that this is an electrolysis process even though a
non-Faraday electrolysis process, that is, a large of organic substances would cause the
decrease of solution conductance to consume the electrical energy. At this moment for the
excess existence of organic substances, the over-heat would make the electrodes molten. In
this work, a ratio of formic acid-to-distilled water (v/v) was used to be 5 : 95.
Table 1 Influence of formic acid dosage on the formation of oxalic acid
Formic
acid : distilled water |
1:99 |
5:95 |
10:90 |
15:85 |
20:80 |
Formation of oxalic acid (g) |
0.0110 |
0.2080 |
0.2513 |
0.2756 |
0.2854 |
Perhaps, for the commonly chemical reaction, the yield of product would increase with increasing reaction time. However, for the reaction caused by plasma was not similar. Just as mentioned above, the attack coming from the energetic species to substances in aqueous solution is no selection. Thus, the formed oxalic acid could also be decomposed into CO2 and H2O. From Table 2, it can be seen that the reaction time of 90 min was enough.
Table 2 Influence of reaction time on the formation of oxalic acid
Reaction time (min) |
60 |
90 |
120 |
150 |
180 |
Formation of oxalic acid (g) |
0.1676 |
0.2080 |
0.2125 |
0.2440 |
0.2675 |
The reaction temperature should be controlled due to being lots of Joule heating during glow discharge process. Otherwise, the electrodes would be destroyed. In fact, the higher temperature is not benefit to formation of oxalic acid, listing in Table 3.
Table 3 Effect of reaction temperature on the formation of oxalic acid
Reaction temperature ( ºC) |
10-15 |
20-25 |
30-35 |
40-45 |
50-55 |
60-65 |
| Formation of oxalic acid (g) | 0.2478 |
0.2140 |
0.2080 |
0.1980 |
0.1807 |
0.1601 |
In conclusion, it may be said that it is possible to synthesize oxalic acid in solid from formic acid by using the glow discharge plasma technique. In appropriate conditions the solid oxalic acid yield of 10.6% was obtained. Based on the data of HPLC, about 48% of formic acid was converted (maybe including degradation itself). The contact glow discharge plasma is, truly, a green energy source, even though it has not been used in practice. A strongly potential ability for application in synthetic chemistry will appear in future.
AcknowledgementsThis work was supported in part by the Project of Key Science and Technology of Education Ministry (00250), the Nature Science Foundation of Gansu Province (3ZS041-A25- 028), the Invention Project of Science & Technology of NWNU (KJCXGC-01), and Gansu Key Lab of Polymer Materials, China.
REFERENCES
[1] A.R. Denaro, A. Hicking, J. Electrochem. Soc., 1958, 105: 265.
[2] J.Z. Gao, A.X. Wang, Y. Fu et al., Plasma Sci. Technol., 2008, 10: 30.
[3] J.Z. Gao, Pakistan J. Biol. Sci., 2006, 9: 323.
[4] K. Harada, T. Iwasaki, Nature, 1974, 250: 426.
[5] K. Harada, S. Suzuki, Nature, 1977, 266: 275.
[6] S.K. Sengupta, R. Singh, A.K. Srivastava, Indian J. Chem., 1995, 34A: 459.
利用接触辉光放电电解等离子体在甲酸溶液中合成草酸的尝试
高锦章,付燕,王爱香,吴建林,李岩,杨武
(西北师范大学 化学化工学院, 730070 兰州)
摘要
接触辉光放电电解等离子体技术在水处理中的研究与应用已有许多报道,相对而言,在合成化学中的应用尚不多见,常见有报道在溶液中检测到的产物,很难分离。由于羟基自由基缺乏选择性,产物的生成与降解两个过程并存。另外,必须考虑到反应物在水中的溶解度,若难溶于水,则很难利用该技术,因为这毕竟属于电解过程,等离子体只能在含电解质的水溶液中产生。由于这是一项绿色能源技术,有待深入研究。本文在不损坏电极与设备的前提下,在稀甲酸溶液中合成并提纯得到了白色结晶草酸,产率达到10.6%,当属一种新的尝试。
关键词 合成 草酸
接触辉光放电电解等离子