http://www.chemistrymag.org/cji/2008/10c057pe.htm |
Dec.
1, 2008 Vol.10 No.12 P.57 Copyright |
Hydrothermal synthesis and crystal structure of a two-dimensional cerium(III) sulfate hydrate, [Ce2(SO4)3 (H2O)4]·H2O and 2,2'-bipyridine-4,4'-dicarboxylic acid
Wang Chongchen, Wang Peng(School of Environmental and Energy Engineering, Beijing University of Civil Engineering and Architecture, Beijing 100044, China)
Received Sep. 16, 2008; Supported by Funding Project for Academic Human Resources Development in Institutions of Higher Learning Under the Jurisdiction of Beijing Municipality(Grant no. BJE10016200611), and the Research Fund of Beijing University of Civil Engineering and Architecture (grant no. 100700502).
Abstract [Ce2(SO4)3 (H2O)4]·H2O (1) and 2,2'-bipyridine-4,4'-dicarboxylic acid (2), were hydrothermally synthesized, and single crystal X-ray diffraction revealed that compound (1) crystallizes in monoclinic, space group C2/c with a=1.5745(2) nm, b= 0.96286(12) nm, c=1.03574(17) nm, b=120.000(2)o and Z=4, and compound (2) crystallizes in Orthorhombic crystal system, space group Fdd2 with a=3.7808(8) nm, b=1.4620(3) nm, c=0.36627(7) nm, and Z=8. The two-dimensional layer of compound (1) was composed of cerium(III) centers, coordination water molecules and [SO4]2- ligands. The cerium(III) center is nine-coordinated by seven oxygen atoms from [SO4]2- units and two oxygen atoms from two coordination water molecules and the [SO4]2- ligands display two different coordination modes. The strong hydrogen bonding interactions between the coordination water molecules, lattice water molecules and [SO4]2- units contribute to further link the 2-D layers into 3-D framework. The result given by thermogravimetric analysis revealed that compound (1) was thermally stable up to 150oC. While the compound (2) remained the typical bond distances and bond angles, and 2-D layer is constructed by O1---H...N1 hydrogen bond.
Keywords cerium; 2,2'-bipyridine-4,4'-dicarboxylic acid; hydrothermal synthesis; crystal structure; thermal property There is considerable current interest in the synthesis and characterization of multi-dimensional coordination polymers because of their applications. Multi-dentate ligands, which are used as building blocks in constructing the coordination polymers are quite important in the crystal engineering of the supramolecular architecture organized by coordinate covalent or hydrogen bonding interaction. 2,2'-bipyridine-4,4'-dicarboxylic acid (bpdc) is a potential bridging ligand in various coordination models as a result of its multi-functional groups. The synthesis, structure and properties on the coordination compound constructed by bpdc and transition and lanthanide metal were investigated [1-7], which revealed that bpdc is versatile organic ligand due to its diverse coordination modes of carboxylate groups. Encouraged by the previous works by others, the hydrothermal reaction between Ce(NO3)3·6H2O, 2,2'-bipyridine-4,4'-dicarboxylic acid and AgSCN designed and carried out. The results gave two compounds, one was two-dimensional inorganic compound [Ce2(SO4)3 (H2O)4]·H2O, and the other is the organic 2,2'-bipyridine-4,4'-dicarboxylic acid ligand. 1 EXPERIMENTAL
Reagent grade chemicals were used as received without further purification. IR spectra were recorded on a Perkin Elmer FT-IR Spectrum Spectrophotomer (Spectrum 100) in region of 400-4000 cm-1 using KBr pellets. TGA was performed from room temperature to 700oC in a N2 stream at a flow rate of 10oC·min-1 on a ZRY-1P TGA system using a-Al2O3 as reference material.
1.1 Synthesis of [Ce2(SO4)3 (H2O)4]·H2O and 2,2'-bipyridine-4,4'-dicarboxylic acid
Colorless block-shaped crystals of [Ce2(SO4)3 (H2O)4]·H2O (1) and colorless block-like crystals of 2,2'-bipyridine-4,4'-dicarboxylic acid (2) were obtained by hydrothermal reaction of Ce(NO3)3·6H2O (0.0434 g), 2,2'-bipyridine-4,4'-dicarboxylic acid (0.0244g), AgSCN (0.0165g)and deionized water (15 mL) in a 23 mL polytetrafluoroethylene (PTFE)-lined stainless steel autoclave reactor at 433 K for 120 h followed by slow cooling to room temperature. The colorless block-like crystals of compound (1) and (2) are so different to separate them from each other, and the yields are ca. 46% and 27% for compound (1) and (2) respectively based on input Ce(NO3)3·6H2O and 2,2'-bipyridine-4,4'-dicarboxylic acid.
1.2 X-ray crystallography
Crystals of compound (1) and (2) suitable for single-crystal X-ray diffraction was selected for the experiment. The intensity data of the title compound were collected at room temperature (293 K) on a Rigaku R-axis Rapid IP Area Detector diffractometer by w Oscillation scan technique using graphite- monochromatized Mo Kα radiation (l=0.071073 nm). For (1), the total reflections of 6371 were measured, unique 1562 with R(int)=0.050 within the limits 3.11o<q<27.54o, and for (2), the total reflections of 4399 were measured, unique 673 with R(int)=0.135 within the limits 3.52o<q<27.50o.The structure was solved using direct method with SHELXS-97 [8] and refined by full-matrix least-squares on F2 with anisotropic thermal parameters for all non-hydrogen atoms. Hydrogen atoms were geometrically fixed to allow riding on the parent atoms to which they are attached. All calculations were performed using SHELXTL-97 program package [9]. For (1), the final full-matrix least-squares refinement gave R1 = 0.0238, wR2 =0.0564. The highest and lowest residual peaks in the final difference Fourier map are 100.8 and -180.6 e/nm3, respectively. The crystal belongs to monoclinic crystal system, space group C2/c with a=1.5745(2) nm, b=0.96286(12) nm, c=1.03574(17) nm, b=120.000(2)o and Z=4. For (2), the final full-matrix least-squares refinement gave R1 = 0.0484, wR2 =0.0999. The highest and lowest residual peaks in the final difference Fourier map are 52.8 and -56.7e/nm3, respectively. The crystal belongs to Orthorhombic crystal system, space group Fdd2 with a=3.7808(8) nm, b=1.4620(3) nm, c=0.36627(7) nm, and Z=8. Table 1 Bond lengths and angles for compound (1)
Bond length/nm |
Bond angle/(o) |
Bond angle/(o) |
Ce(1)-O(3)#1 2.426(3) |
O(3)#1-Ce(1)-O(5)#2 82.89(10) |
O(2)#3-Ce(1)-O(6)#4 77.64(9) |
2.1 The synthesis of compound (1) and (2)
The hydrothermal reaction between Ce(NO3)3·6H2O, AgSCN and 2,2'-bipyridine-4,4'- dicarboxylic acid failed to produce the organic-inorganic hybrid compound containing cerium centers and 2,2'-bipyridine-4,4'-dicarboxylic acid ligands, but gave a novel inorganic compound and the organic ligand itself. More experiments to test the role of AgSCN, but the results revealed that the title compounds were failed to obtain without AgSCN. More tries to replace AgSCN with Ag2(SO4) also failed to harvest the title compounds. It was thought that the hydrolysis of SCN- results into the occurrence of [SO4]2- under the extreme hydrothermal condition.
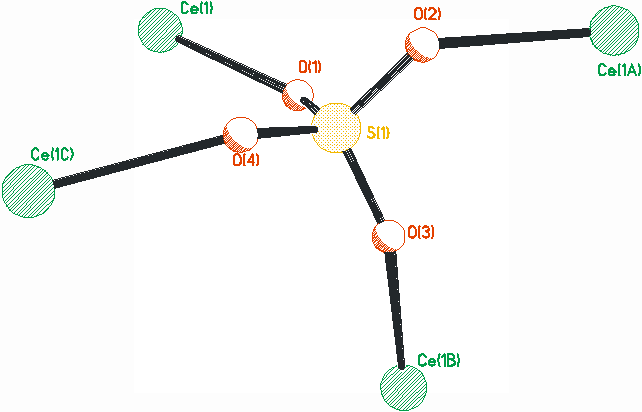
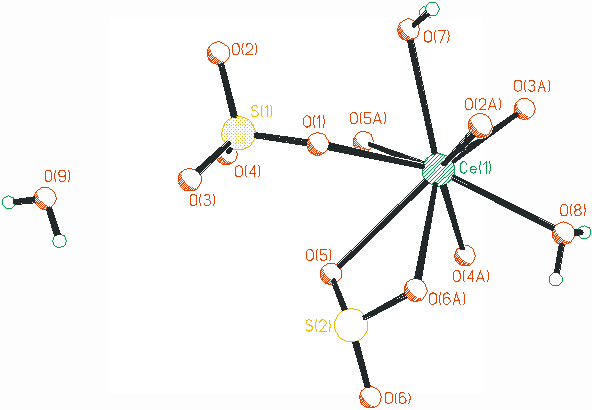 Fig. 1
The asymmetric unit of compound (1)
2.2 The crystal
structure of compound (1)
Fig. 1
The asymmetric unit of compound (1)
2.2 The crystal
structure of compound (1)
The framework of the compound (1) is constructed by CeO9 tetradecahedra and SO4 tetrahedra. The existence of [SO4]2- can be proved by the strong character peak at 1090 cm-1. The cerium center is nine-coordinated by five oxygen atoms from four [SO4]2- anions via mono-coordination mode, two oxygen atoms from a [SO4]2- unit by chelating mode, and two oxygen atoms from two water molecules, as shown in Fig.1. There are no apparent difference between the bond distance for the Ce-O([SO4]2-) and Ce-O(H2O), ranging from 0.2426 to 0.2619 nm, except for the Ce-O(5) 0.2807 nm. The bond angle of O-Ce-O ranges from 51.68o to 142.91o, as listed in Table 1, which are similar to other cerium sulfate reported [10-12].
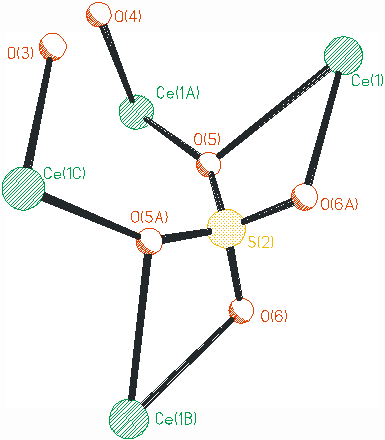
In the title compound, the [SO4]2- anions show two different coordination modes to link the cerium centers, as illustrated in Fig. 2. The [S1O4]2- is tetra(mono-dentate) ligand to join four different cerium centers. While the [S2O4]2- acts as hexa-dentate ligand to link four cerium ions, in which the O(5) and O(6A) are coordinated to one cerium center in chelating mode, and the O(5) is also linked to another cerium via mono-dentate mode. In the title compound, the [SO4]2- units play the role of multi-dentate ligands, and also to balance the charge of the whole compound.
|
|
|
|
Table 2 Hydrogen bonds (nm and o) in the crystal structure of compound (1)
D-H d(D-H) d(H..A) <DHA d(D..A) A ( Symmetry transformation) |
O7-H7B 0.850 2.209 157.82 3.014 O1 [ x, -y+1, z-1/2 ] |
2.3 The crystal structure of compound
(2)
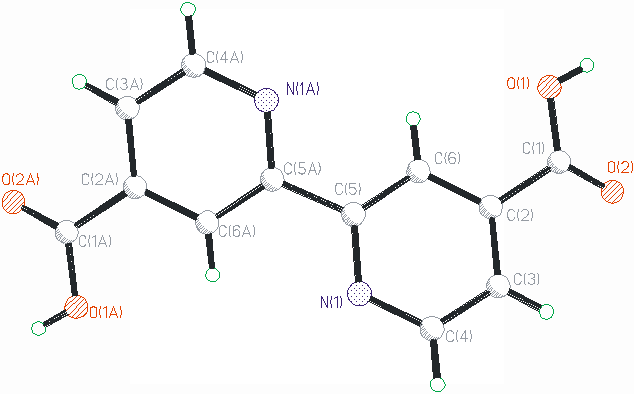
The crystal structure of 2,2'-bipyridine-4,4'-dicarboxylic acid, as shown in Fig. 4, in
which the two pyridyl rings in the same 2,2'-bipyridine-4,4'-dicarboxylic acid are not in
the same plane. The 2-D framework of the title compound built from
2,2'-bipyridine-4,4'-dicarboxylic acid molecules linked by O1---H...N1 hydrogen bond, as
listed in Table 3. The reported crystal structure crystilized in monoclinic with space
group of Cc. Our present work under room temperature (293K) is quite different from the
previously work
D-H d(D-H) d(H..A) <DHA d(D..A) A ( Symmetry transformation) |
O1-H1B 0.82 1.85 166 2.652(4) N1 [ x, y+1/2, z-1/2 ] |
In order to study the thermal stability of the title compound, the thermaogravimetric analysis was carried out in nitrogen. The result showed (Fig. 4) that from room temperature to ca. 150 oC, 2.80% weight loss is approximately the weight of one lattice water molecule (cal. 2.73%). From 150 oC to 350 oC, 11.5% weight loss corresponds to the loss of four coordination water molecules (cal. 10.9%). Above 650 oC, the material lost weight up to 11.9 % equivalent to the loss of SO3 (cal. 12.1%). The residual of the thermal treatment is Ce2O(SO4)2, and the existence of SO4 unit can be proved by the character peak at 1130 cm-1.
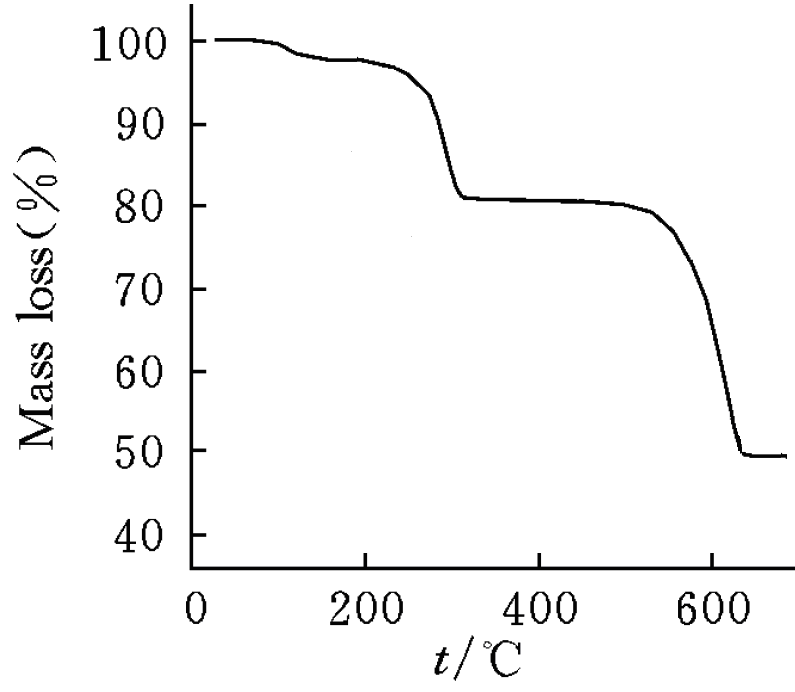
Fig. 5 TG curve of the compound (1)
REFERENCES
[1] Schareina T, Schick C, Kempe R. Z. Anorg. Allg. Chem., 627, 2001: 131.
[2] Schareina T, Schick C, Abrahams B et al. Z. Anorg. Allg. Chem., 627, 2001: 1711.
[3] Eskelinen E, Luukkanen S, Haukka M et al. J. Chem. Soc. Dalton Trans., 2000: 2745.
[4] Tynan E, Jensen P, Kruger P E et al. Chem. Commun., 2004: 776.
[5] Tynan E, Jensen P, Kelly N R et al. Dalton Trans., 2004: 3440.
[6] Liu Y, Lu Y, Wu H et al. Inorg. Chem., 41, 2001: 2592.
[7] Wu J, Yeh T, Wen Y et al. Cryst. Growth Des., 6, 2006: 467.
[8] Sheldrick G M SHELXS-97, Program for X-ray Crystal Structure Solution. University of Göttingen: Germany, 1997.
[9] Sheldrick G M SHELXL-97, Program for the Refinement of Crystal Structures. University of Göttingen: Germany, 1997
[10] Xin M-H, Wang Y, Zhu G.-S et al. Chem. J. Chinese U., 26, 2005: 2007.
[11] Xu X. Acta Crystallogr., E61, 2008: i1.
[12] Dereigne A, Manoli J, Pannetier G. Bull Soc Fr Mineral Cristallogr., 95, 1972: 269.
二维硫酸亚铈水合物[Ce2(SO4)3 (H2O)4]·H2O与2,2'-联吡啶-4,4'-二甲酸的水热合成与晶体结构
王崇臣, 王鹏
(北京建筑工程学院 环境与能源工程学院, 北京 100044)
摘要 通过水热合成法获得了[Ce2(SO4)3 (H2O)4]·H2O(1) 和2,2'-联吡啶-4,4'-二甲酸(2)。X-单晶衍射结果表明,化合物(1)属于单斜晶系,空间群为C2/c,晶胞参数为a=1.5745(2) nm、b= 0.96286(12) nm、c=1.03574(17) nm、b=120.000(2)o、Z=4, 而化合物(2)属于正交晶系,空间群为Fdd2,晶胞参数为a=3.7808(8) nm、b=1.4620(3) nm、c=0.36627(7) nm、Z=8。化合物(1)的二维无机层由中心离子铈(III)、水分子以及硫酸根组成,其中铈(III)和7个来自硫酸根的氧原子以及2个来自配位水分子的氧原子配位,而硫酸根呈现出两种截然不同的配位模式。该化合物中的配位水分子、结晶水以及硫酸根间的丰富氢键进一步将二维无机层连接成三维结构。热分析结果表明该化合物在150 oC以下保持热稳定。化合物(2)保持了典型的键长和键角,并且由氢键作用构建了二维结构。
关键词 铈; 2,2'-联吡啶-4,4'-二甲酸; 水热合成; 晶体结构; 热性能